Temporal lobe epilepsy
| Temporal lobe epilepsy | |
|---|---|
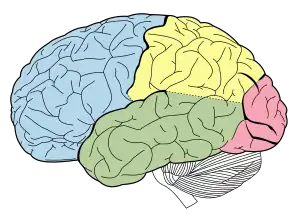 | |
| Lobes of the brain. Temporal lobe in green | |
| Specialty | Neurology, Psychiatry |
Temporal lobe epilepsy (TLE) is a chronic disorder of the nervous system which is characterized by recurrent, unprovoked focal seizures that originate in the temporal lobe of the brain and last about one or two minutes. TLE is the most common form of epilepsy with focal seizures.[1] A focal seizure in the temporal lobe may spread to other areas in the brain when it may become a focal to bilateral seizure.
TLE is diagnosed by taking a medical history, blood tests, and brain imaging. It can have a number of causes such as head injury, stroke, brain infections, structural lesions in the brain, brain tumors, or it can be of unknown onset. The first line of treatment is through anticonvulsants. Surgery may be an option, especially when there is an observable abnormality in the brain. Another treatment option is electrical stimulation of the brain through an implanted device called the vagus nerve stimulator (VNS).[1]
Types
Over forty types of epilepsy are recognized and these are divided into two main groups: focal seizures and generalized seizures.[2] Focal seizures account for approximately sixty percent of all adult cases.[3] Temporal lobe epilepsy (TLE) is the single most common form of focal seizure.[4]
The International League Against Epilepsy (ILAE) recognizes two main types of temporal lobe epilepsy: mesial temporal lobe epilepsy (MTLE), arising in the hippocampus, the parahippocampal gyrus and the amygdala which are located in the inner (medial) aspect of the temporal lobe, and lateral temporal lobe epilepsy (LTLE), the rarer type, arising in the neocortex at the outer (lateral) surface of the temporal lobe.[3] The seizures of LTLE are characterized by auditory or visual features. Autosomal dominant lateral temporal lobe epilepsy (ADLTLE) is a rare hereditary condition, often associated with mutations in the LGI1 gene.[5]
Signs and symptoms
When a seizure begins in the temporal lobe, its effects depend on the precise location of its point of origin, its locus. In 1981, the ILAE recognized three types of seizures occurring in temporal lobe epilepsy. The classification was based on EEG findings.[6] However, as of 2017 the general classification of seizures has been revised.[7] The newer classification uses three key features: where the seizures begin, the level of awareness during a seizure, and other features.[7]
Focal seizures
Focal seizures in the temporal lobe involve small areas of the lobe such as the amygdala and hippocampus.
The newer classification gives two types of focal onset seizures, as focal aware and focal impaired awareness.[2]
Focal aware seizures
Focal aware means that the level of consciousness is not altered during the seizure.[2] In temporal lobe epilepsy, a focal seizure usually causes abnormal sensations only. Often, the patient cannot describe the sensations.[8]
These may be:
- Sensations such as déjà vu (a feeling of familiarity), jamais vu (a feeling of unfamiliarity)
- Amnesia of a single memory or set of memories
- A sudden sense of unprovoked fear and anxiety
- Nausea
- Auditory, visual, olfactory, gustatory, or tactile hallucinations; olfactory hallucinations often seem indescribable to patients beyond "pleasant" or "unpleasant"[8]
- Visual distortions such as macropsia and micropsia
- Dissociation or derealisation
- Synesthesia (stimulation of one sense experienced in a second sense)[9]
- Dysphoric or euphoric feelings, fear, anger, and other emotions
Focal aware seizures are often called "auras" when they serve as a warning sign of a subsequent seizure. Regardless, an aura is actually a seizure itself, and such a focal seizure may or may not progress to a focal impaired awareness seizure.[10] People who experience only focal aware seizures may not recognize what they are, nor seek medical care.
Focal impaired awareness seizures
Focal impaired awareness seizures are seizures which impair consciousness to some extent:[2] they alter the person's ability to interact normally with their environment. They usually begin with a focal aware seizure, then spread to a larger portion of the temporal lobe, resulting in impaired consciousness. They may include autonomic and psychic features present in focal aware seizures.
Signs may include:[11]
- Motionless staring
- Automatic movements of the hands or mouth
- Confusion and disorientation
- Altered ability to respond to others, unusual speech
- Transient aphasia (losing ability to speak, read, or comprehend spoken word)
These seizures tend to have a warning or aura before they occur, and when they occur they generally tend to last only 1–2 minutes. It is not uncommon for an individual to be tired or confused for up to 15 minutes after a seizure has occurred, although postictal confusion can last for hours or even days. Though they may not seem harmful, due to the fact that the individual does not normally seize, they can be extremely harmful if the individual is left alone around dangerous objects or in situations requiring acute awareness, such as sterring a vehicle or operating machinery. With this type, some people do not even realize they are having a seizure and most of the time their memory from right before to after the seizure is wiped. First-aid is only required if there has been an injury or if this is the first time a person has had a seizure.
Focal to bilateral seizures or generalized seizures
Seizures which begin in the temporal lobe, and then spread to involve both sides of the brain are termed focal to bilateral. Where both sides of the brain or the whole brain are involved from the onset, these seizures are known as generalized seizures and may be tonic clonic.[7] The arms, trunk, and legs stiffen (the tonic phase), in either a flexed or extended position, and then jerk (the clonic phase). These were previously known as grand mal seizures.[11] The word grand mal comes from the French term, meaning major affliction.
Postictal period
There is some period of recovery in which neurological function is altered after each of these seizure types. This is the postictal state. The degree and length of postictal impairment directly correlates with the severity of the seizure type. Focal aware seizures often last less than sixty seconds; focal with impaired awareness seizures may last up to two minutes; and generalized tonic clonic seizures may last up to three minutes. The postictal state in seizures other than focal aware may last much longer than the seizure itself.
Because a major function of the temporal lobe is short-term memory, a focal with impaired awareness seizure, and a focal to bilateral seizure can cause amnesia for the period of the seizure, meaning that the seizure may not be remembered.
Complications
Depression
Individuals with temporal lobe epilepsy have a higher prevalence of depression than the general population. Although the psychosocial impacts of epilepsy may be causative, there are also links in the phenomenology and neurobiology of TLE and depression.[12]
Memory
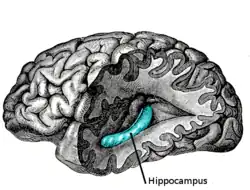
The temporal lobe and particularly the hippocampus play an important role in memory processing. Declarative memory (memories which can be consciously recalled) is formed in the area of the hippocampus called the dentate gyrus.
Temporal lobe epilepsy is associated with memory disorders and loss of memory. Animal models and clinical studies show that memory loss correlates with temporal lobe neuronal loss in temporal lobe epilepsy. Verbal memory deficit correlates with pyramidal cell loss in TLE. This is more so on the left in verbal memory loss. Neuronal loss on the right is more prominent in non-verbal (visuospatial memory loss).[13][14][15][16][17]
Childhood onset
After childhood onset, one third will "grow out" of TLE, finding a lasting remission up to an average of 20 years. The finding of a lesion such as hippocampal sclerosis (a scar in the hippocampus), tumour, or dysplasia, on magnetic resonance imaging (MRI) predicts the intractability of seizures.[18]
Personality
The effect of temporal lobe epilepsy on personality is a historical observation dating to the 1800s. Personality and behavioural change in temporal lobe epilepsy is seen as a chronic condition when it persists for more than three months.
Geschwind syndrome is a set of behavioural phenomena seen in some people with TLE. Documented by Norman Geschwind, signs include: hypergraphia (compulsion to write (or draw) excessively), hyperreligiosity (intense religious or philosophical experiences or interests), hyposexuality (reduced sexual interest or drive), circumstantiality (result of a non-linear thought pattern, talks at length about irrelevant and trivial details).[19] The personality changes generally vary by hemisphere.[19]
The existence of a "temporal lobe epileptic personality" and of Geschwind syndrome have been disputed and research is inconclusive.[19]
Causes
The causes of TLE include mesial temporal sclerosis, traumatic brain injury, brain infections, such as encephalitis and meningitis, hypoxic brain injury, stroke, cerebral tumours, and genetic syndromes. Temporal lobe epilepsy is not the result of psychiatric illness or fragility of the personality.[11]
Febrile seizures
Although the theory is controversial, there is a link between febrile seizures (seizures coinciding with episodes of fever in young children) and subsequent temporal lobe epilepsy, at least epidemiologically.[20][21][22][23]
Human herpes virus 6
In the mid-1980s, human herpesvirus 6 (HHV-6) was suggested as a possible causal link between febrile convulsions and mesial temporal lobe epilepsy. However, although the virus is found in temporal lobe tissue at surgery for TLE, it has not been recognised as a major factor in febrile seizures or TLE.[24][25][26]
Reelin
Dispersion of the granule cell layer in the hippocampal dentate gyrus is occasionally seen in temporal lobe epilepsy and has been linked to the downregulation of reelin, a protein that normally keeps the layer compact by containing neuronal migration. It is unknown whether changes in reelin expression play a role in epilepsy.[27][28]
Pathophysiology
Neuronal loss
In TLE, there is loss of neurons in region CA1 and CA3 of the hippocampus.[29][30] There is also damage to mossy cells and inhibitory interneurons in the hilar region of the hippocampus (region IV) and to the granule cells of the dentate gyrus. In animal models, neuronal loss occurs during seizures but in humans, neuronal loss predates the first seizure and does not necessarily continue with seizure activity.[31][32][33][34][35] The loss of the GABA-mediated inhibitory interneurons may increase the hyperexcitability of neurons of the hippocampus leading to recurrent seizures.[36] According to the "dormant basket cell" hypothesis, mossy cells normally excite basket cells which in turn, inhibit granule cells. Loss of mossy cells lowers the threshold of action potentials of the granule cells.[37]
GABA reversal
In certain patients with temporal lobe epilepsy it has been found that the subiculum could generate epileptic activity. It has been found that GABA reversal potential is depolarising[38] in the subpopulation of the pyramidal cells due to the lack of KCC2 co-transporter. It has been shown that it is theoretically possible to generate seizures in the neural networks due to down-regulation of KCC2,[39] consistent with the chloride measurements during the transition to seizure[40] and KCC2 blockade experiments.[41]
Granule cell dispersion in the dentate gyrus
Granule cell dispersion is a type of developmental migration and a pathological change found in the TLE brain which was first described in 1990.[42][43] The granule cells of the dentate gyrus are tightly packed forming a uniform, laminated layer with no monosynaptic connections.[44] This structure provides a filter for the excitability of neurons.[44]
In TLE, granule cells are lost, the structure is no longer closely packed and there are changes in the orientation of dendrites.[43][45] These changes may or may not be epileptogenic. For instance, if the dendrites of granule cells reconnect, it may be in a way (through the laminar planes) that allows hyperexcitability.[32] However, not all patients have granule cell dispersion.[29]: 387–389
Aberrant mossy fiber sprouting
Mossy fibers are the axons of granule cells. They project into the hilus of the dentate gyrus and stratum lucidum in the CA3 region giving inputs to both excitatory and inhibitory neurons.[44][46][47]
In the TLE brain, where granule cells are damaged or lost, axons, the mossy fibres, 'sprout' in order to reconnect to other granule cell dendrites. This is an example of synaptic reorganization. This was noted in human tissue in 1974 and in animal models in 1985. In TLE, the sprouting mossy fibres are larger than in the normal brain and their connections may be aberrant. Mossy fibre sprouting continues from one week to two months after injury.[29]: 416–431 [44][48][49][50]
Aberrant mossy fibre sprouting may create excitatory feedback circuits that lead to temporal lobe seizures. This is evident in intracellular recordings.[51] Stimulation of aberrant mossy fibre areas increases the excitatory postsynaptic potential response.[52][53]
However, aberrant mossy fiber sprouting may inhibit excitatory transmission by synapsing with basket cells which are inhibitory neurons and by releasing GABA and neuropeptide Y which are inhibitory neurotransmitters. Also, in animal models, granule cell hyper-excitability is recorded before aberrant mossy fibre sprouting has occurred.[54][55][56][57]
Diagnosis
.png.webp) Memory encoding activations in controls, left temporal lobe epilepsy individuals, and right temporal lobe epilepsy individuals
Memory encoding activations in controls, left temporal lobe epilepsy individuals, and right temporal lobe epilepsy individuals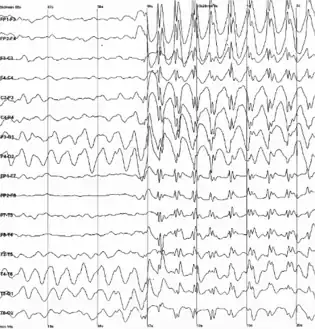 Epileptic spike and wave discharges monitored with EEG
Epileptic spike and wave discharges monitored with EEG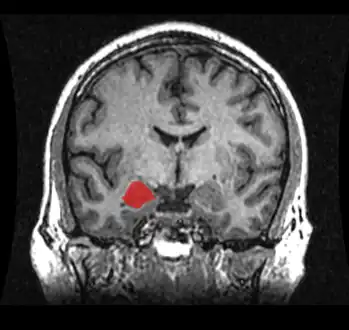 T1 weighted MRI scan of a normal coronal section of human brain with amygdala marked in red.
T1 weighted MRI scan of a normal coronal section of human brain with amygdala marked in red.
The diagnosis of temporal lobe epilepsy can include the following methods: Magnetic resonance imaging (MRI), CT scans, positron emission tomography (PET), EEG, and magnetoencephalography.[58]
Imaging
CT scan is useful as the emergency situations when the suspected cause of epilepsy is due to conditions such as intracerebral haemorrhage, or brain abscesses, or when MRI imaging is not readily available or there is any contraindications to MRI such as the presence of cardiac pacemakers or cochlear implants in the subject's body. CT scan also detect some abnormal calcifications in the brain that is characteristic of diseases such as tuberous sclerosis and Sturge–Weber syndrome. However, CT scan is not sensitive or specific enough when compared in MRI in detecting the common causes of epilepsy such as small tumours, vascular malformations, abnormalities of cerebral cortex development, or abnormalities in the medial part of the temporal lobe.[59]
MRI is the imaging choice when assessing those with epilepsy. In newly diagnosed epilepsy, MRI can detect brain lesion in up to 12 to 14% of the cases. However, for those with chronic epilepsy, MRI can detect brain lesion in 80% of the cases. However, in cases where there is definite clinical and EEG diagnosis of idiopathic generalized epilepsy, or Rolandic epilepsy, MRI scan is not needed.[59]
Differential diagnosis
Other medical conditions with similar symptoms include panic attacks, psychosis spectrum disorders, tardive dyskinesia, and occipital lobe epilepsy.[60]
Treatments
Anticonvulsants
Many anticonvulsant oral medications are available for the management of temporal lobe seizures. Most anticonvulsants function by decreasing the excitation of neurons, for example, by blocking fast or slow sodium channels or by modulating calcium channels; or by enhancing the inhibition of neurons, for example by potentiating the effects of inhibitory neurotransmitters like GABA.
In TLE, the most commonly used older medications are phenytoin, carbamazepine, primidone, valproate, and phenobarbital. Newer drugs, such as gabapentin, topiramate, levetiracetam, lamotrigine, pregabalin, tiagabine, lacosamide, and zonisamide promise similar effectiveness, with possibly fewer side-effects. Felbamate and vigabatrin are newer, but can have serious adverse effects so they are not considered as first-line treatments.
Up to one third of patients with medial temporal lobe epilepsy will not have adequate seizure control with medication alone. For patients with medial TLE whose seizures remain uncontrolled after trials of several types of anticonvulsants (that is, the epilepsy is intractable), surgical excision of the affected temporal lobe may be considered.[61]
Surgical interventions
Epilepsy surgery has been performed since the 1860s and doctors have observed that it is highly effective in producing freedom from seizures. However, it was not until 2001 that a scientifically sound study was carried out to examine the effectiveness of temporal lobectomy.[62]
Temporal lobe surgery can be complicated by decreased cognitive function. However, after temporal lobectomy, memory function is supported by the opposite temporal lobe; and recruitment of the frontal lobe.[63][64] Cognitive rehabilitation may also help.[65]
Other treatments
Where surgery is not recommended, further management options include new (including experimental) anticonvulsants, and vagus nerve stimulation. The ketogenic diet is also recommended for children, and some adults.[66] Other options include brain cortex responsive neural stimulators, deep brain stimulation, stereotactic radiosurgery, such as the gamma knife, and laser ablation.[67]
Effects on society
The first to record and catalog the abnormal symptoms and signs of TLE was Norman Geschwind. He found a constellation of symptoms that included hypergraphia, hyperreligiosity, collapse, and pedantism, now called Geschwind syndrome.
Vilayanur S. Ramachandran explored the neural basis of the hyperreligiosity seen in TLE using the galvanic skin response (GSR), which correlates with emotional arousal, to determine whether the hyperreligiosity seen in TLE was due to an overall heightened emotional state or was specific to religious stimuli. Ramachandran presented two subjects with neutral, sexually arousing and religious words while measuring GSR. Ramachandran was able to show that patients with TLE showed enhanced emotional responses to the religious words, diminished responses to the sexually charged words, and normal responses to the neutral words. This study was presented as an abstract at a neuroscience conference and referenced in Ramachandran's book, Phantoms in the Brain,[68] but it has never been published in the peer-reviewed scientific press.[69]
A study in 2015, reported that intrinsic religiosity and religiosity outside of organized religion were higher in patients with epilepsy than in controls.[70] Lower education level, abnormal background EEG activity, and hippocampal sclerosis have been found to be contributing factors for religiosity in TLE.[71]
TLE has been suggested as a materialistic explanation for the revelatory experiences of prominent religious figures such as Abraham, Moses, Jesus, Mohammed, Saint Paul, Joan of Arc,[72] Saint Teresa of Ávila, and Joseph Smith. These experiences are described (in possibly unreliable accounts) as complex interactions with their visions; but possibly (and dependent on the reliability of historical accounts, often made by acolytes) lack the stereotypy, amnestic periods, and automatisms or generalized motor events, which are characteristic of TLE. Psychiatric conditions with psychotic spectrum symptoms might be more plausible physical explanation of these experiences.[73] It has been suggested that Pope Pius IX's doctrine of the immaculate conception was influenced by his forensically diagnosed partial epilepsy.[74]
In 2016, a case history found that a male temporal lobe epileptic patient experienced a vision of God following a temporal lobe seizure, while undergoing EEG monitoring. The patient reported that God had sent him to the world to "bring redemption to the people of Israel".[75] The purported link between TLE and religiosity has inspired work by Michael Persinger and other researchers in the field of neurotheology. Others have questioned the evidence for a link between temporal lobe epilepsy and religiosity.[69][76]
References
- 1 2 NINDS (1 February 2016), The Epilepsies and Seizures: Hope Through Research, National Institute of Neurological Disorders and Stroke (NINDS), U.S. National Institutes of Health (NIH), archived from the original on 27 July 2016, retrieved 8 August 2016
- 1 2 3 4 "Types of Seizures". Epilepsy Foundation. Archived from the original on 22 December 2017.
- 1 2 Engel J (2001). "A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology". Epilepsia. 42 (6): 796–803. doi:10.1046/j.1528-1157.2001.10401.x. PMID 11422340.
- ↑ Wiebe S (May 2000). "Epidemiology of temporal lobe epilepsy". Can J Neurol Sci. 27 Suppl 1: S6–10, discussion S20–1. doi:10.1017/s0317167100000561. PMID 10830320.
- ↑ Nobile, C; Michelucci, R; Andreazza, S; Pasini, E; Tosatto, SC; Striano, P (April 2009). "LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy". Human Mutation. 30 (4): 530–6. doi:10.1002/humu.20925. PMID 19191227.
- ↑ ILAE (1981). "Proposal for revised clinical and electroencephalographic classification of epileptic seizures". Epilepsia. 22 (4): 489–501. doi:10.1111/j.1528-1157.1981.tb06159.x. PMID 6790275. S2CID 22190102.
- 1 2 3 "2017 Revised Classification of Seizures". epilepsy.com. Epilepsy Foundation. Archived from the original on 22 December 2017.
- 1 2 Sacks, Oliver (2012). Hallucinations. Knopf. pp. 144. ISBN 978-0307957245.
- ↑ Neckar, Marcel; Bob, Petr (11 January 2016). "Synesthetic associations and psychosensory symptoms of temporal epilepsy". Neuropsychiatric Disease and Treatment. 12: 109–12. doi:10.2147/NDT.S95464. PMC 4714732. PMID 26811683.
- ↑ "Simple partial seizures". hopkinsmedicine.org. Johns Hopkins Medicine. Archived from the original on 3 February 2014. Retrieved 2 February 2014.
- 1 2 3 "Temporal lobe epilepsy". MayoClinic.org. Archived from the original on 23 May 2016. Retrieved 2 February 2014.
- ↑ Butler T, et al. (2012). "Cortical thickness abnormalities associated with depressive symptoms in temporal lobe epilepsy". Epilepsy & Behavior. 23 (1): 64–67. doi:10.1016/j.yebeh.2011.10.001. PMC 3259282. PMID 22099527.
- ↑ Sutula T, et al. (2002). "Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits". Do seizures damage the brain. Progress in Brain Research. Vol. 135. pp. 95–110. doi:10.1016/S0079-6123(02)35010-6. ISBN 9780444508140. PMID 12143373.
- ↑ Sass K. J.; et al. (1992). "Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability". Journal of Clinical & Experimental Neuropsychology. 14 (5): 662–672. doi:10.1080/01688639208402854. PMID 1474137.
- ↑ Babb T, et al. (1993). "Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy". Archives of Neurology. 50 (8): 812–817. doi:10.1001/archneur.1993.00540080023008. PMID 8352666.
- ↑ Sass K. J.; et al. (1990). "Verbal memory impairment correlates with hippocampal pyramidal cell density". Neurology. 40 (11): 1694–1697. doi:10.1212/wnl.40.11.1694. PMID 2234424. S2CID 46432362.
- ↑ Reminger S. L.; et al. (2004). "Bilateral hippocampal volume predicts verbal memory function in temporal lobe epilepsy". Epilepsy & Behavior. 5 (5): 687–695. doi:10.1016/j.yebeh.2004.06.006. PMID 15380120. S2CID 9117179.
- ↑ Spooner, C. G. (2006). "New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome". Neurology. 67 (12): 2147–2153. doi:10.1212/01.wnl.0000248189.93630.4f. PMID 17082466. S2CID 1238402.
- 1 2 3 Mellers J (2012). "6 Epilepsy". In David A, David AS, Fleminger S, Kopelman M, Lovestone S, Mellers J (eds.). Lishman's Organic Psychiatry: A Textbook of Neuropsychiatry (4th ed.). West Sussex, UK: John Wiley & Sons. pp. 347–348. ISBN 9780470675076.
- ↑ Shinnar S, et al. (2008). "Phenomenology of prolonged febrile seizures: results of the FEBSTAT study". Neurology. 71 (3): 170–176. doi:10.1212/01.wnl.0000310774.01185.97. PMID 18525033. S2CID 9732645.
- ↑ Tarkka R, et al. (2003). "Febrile seizures and mesial temporal sclerosis: no association in a long-term follow-up study". Neurology. 60 (2): 215–218. doi:10.1212/01.WNL.0000037482.55894.B1. PMID 12552033. S2CID 39346417.
- ↑ Berg A. T.; et al. (1999). "Childhood-onset epilepsy with and without preceding febrile seizures". Neurology. 53 (8): 1742–1748. doi:10.1212/WNL.53.8.1742. PMID 10563622. S2CID 5656108.
- ↑ Provenzale J. M.; et al. (2008). "Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis". Am J Roentgenol. 190 (4): 976–983. doi:10.2214/AJR.07.2407. PMID 18356445.
- ↑ Karatas H, Gurer G, Pinar A, et al. (January 2008). "Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis". J. Neurol. Sci. 264 (1–2): 151–6. doi:10.1016/j.jns.2007.08.010. PMID 17804017. S2CID 6390677.
- ↑ Fotheringham J.; et al. (2007). "Association of Human Herpesvirus-6B with Mesial Temporal Lobe Epilepsy". PLOS Med. 4 (5): e180. doi:10.1371/journal.pmed.0040180. PMC 1880851. PMID 17535102.
- ↑ Donati D, et al. (2003). "Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections". Neurology. 61 (10): 1405–1411. doi:10.1212/01.WNL.0000094357.10782.F9. PMC 4294224. PMID 14638964.
- ↑ Haas C. A.; et al. (2002). "Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy". J. Neurosci. 22 (14): 5797–6802. doi:10.1523/JNEUROSCI.22-14-05797.2002. PMC 6757930. PMID 12122039.
- ↑ Heinrich C, et al. (2006). "Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus". J. Neurosci. 26 (17): 4701–4713. doi:10.1523/JNEUROSCI.5516-05.2006. PMC 6674063. PMID 16641251.
- 1 2 3 de Lanerolle N. C. and Noebels J. L. (ed.) Jasper's basic mechanisms of the epilepsies: histopathology of human epilepsy. Oxford University Press 2012 chapter 30 ISBN 978-0-19-974654-5.: 387–389
- ↑ Liu Z, et al. (1994). "Quantitative evaluation of neuronal loss in the dorsal hippocampus in rats with long-term pilocarpine seizures". Epilepsy Research. 17 (3): 237–247. doi:10.1016/0920-1211(94)90054-x. PMID 8013446. S2CID 43597098.
- ↑ Blümcke I, et al. (2000). "Loss of hilar mossy cells in Ammon's horn sclerosis". Epilepsia. 40 (6): 174–180. doi:10.1111/j.1528-1157.2000.tb01577.x. PMID 10999540.
- 1 2 Sloviter R. S. (1987). "Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy". Science. 235 (4784): 73–76. Bibcode:1987Sci...235...73S. doi:10.1126/science.2879352. PMID 2879352.
- ↑ Kobayashi M.; Buckmaster P. S (2003). "Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy". Journal of Neuroscience. 23 (6): 2440–2452. doi:10.1523/JNEUROSCI.23-06-02440.2003. PMC 6741996. PMID 12657704.
- ↑ Bouilleret V, et al. (1999). "Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy". Neuroscience. 89 (3): 717–729. doi:10.1016/s0306-4522(98)00401-1. PMID 10199607. S2CID 37671452.
- ↑ Thorn M, et al. (2005). "Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss". Brain. 128 (6): 1344–1357. doi:10.1093/brain/awh475. PMID 15758032.
- ↑ Meldrum B (1989). "GABAergic mechanisms in the pathogenesis and treatment of epilepsy". British Journal of Clinical Pharmacology. 1: 3–11. doi:10.1111/j.1365-2125.1989.tb03454.x. PMC 1379672. PMID 2667605.
- ↑ Sloviter R. S.; et al. (2003). "Dormant basket cell" hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat". Journal of Comparative Neurology. 459 (1): 44–76. doi:10.1002/cne.10630. PMID 12629666. S2CID 36798455.
- ↑ Huberfeld, Gilles; Wittner, Lucia; Clemenceau, Stéphane; Baulac, Michel; Kaila, Kai; Miles, Richard; Rivera, Claudio (12 September 2007). "Perturbed Chloride Homeostasis and GABAergic Signaling in Human Temporal Lobe Epilepsy". Journal of Neuroscience. 27 (37): 9866–9873. doi:10.1523/JNEUROSCI.2761-07.2007. ISSN 0270-6474. PMC 6672644. PMID 17855601.
- ↑ Buchin, Anatoly; Chizhov, Anton; Huberfeld, Gilles; Miles, Richard; Gutkin, Boris S. (16 November 2016). "Reduced Efficacy of the KCC2 Cotransporter Promotes Epileptic Oscillations in a Subiculum Network Model". Journal of Neuroscience. 36 (46): 11619–11633. doi:10.1523/JNEUROSCI.4228-15.2016. ISSN 0270-6474. PMC 6231544. PMID 27852771.
- ↑ Lillis, Kyle P.; Kramer, Mark A.; Mertz, Jerome; Staley, Kevin J.; White, John A. (1 September 2012). "Pyramidal cells accumulate chloride at seizure onset". Neurobiology of Disease. 47 (3): 358–366. doi:10.1016/j.nbd.2012.05.016. PMC 3392473. PMID 22677032.
- ↑ Sivakumaran, Sudhir; Cardarelli, Ross A.; Maguire, Jamie; Kelley, Matt R.; Silayeva, Liliya; Morrow, Danielle H.; Mukherjee, Jayanta; Moore, Yvonne E.; Mather, Robert J. (27 May 2015). "Selective Inhibition of KCC2 Leads to Hyperexcitability and Epileptiform Discharges in Hippocampal Slices and in Vivo". Journal of Neuroscience. 35 (21): 8291–8296. doi:10.1523/JNEUROSCI.5205-14.2015. ISSN 0270-6474. PMC 4444547. PMID 26019342.
- ↑ Thom M, et al. (2005). "Cell proliferation and granule cell dispersion in human hippocampal sclerosis". J Neuropathol Exp Neurol. 64 (3): 194–201. doi:10.1093/jnen/64.3.194. PMID 15804050.
- 1 2 Houser C. R. (1990). "Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy". Brain Research. 535 (2): 195–204. doi:10.1016/0006-8993(90)91601-c. PMID 1705855. S2CID 7510030.
- 1 2 3 4 Nadler J. V. (2003). "The recurrent mossy fiber pathway of the epileptic brain". Neurochemical Research. 28 (11): 1649–1658. doi:10.1023/a:1026004904199. PMID 14584819. S2CID 2566342.
- ↑ Freimanr T. M.; et al. (2011). "Granule cell dispersion in temporal lobe epilepsy is associated with changes in dendritic orientation and spine distribution". Experimental Neurology. 229 (2): 332–338. doi:10.1016/j.expneurol.2011.02.017. PMID 21376037. S2CID 6207296.
- ↑ Lim C, et al. (1997). "Connections of the hippocampal formation in humans: I. The mossy fiber pathway". Journal of Comparative Neurology. 385 (3): 325–351. doi:10.1002/(sici)1096-9861(19970901)385:3<325::aid-cne1>3.0.co;2-5. PMID 9300763. S2CID 23038930.
- ↑ Henze D. A.; et al. (2000). "The multifarious hippocampal mossy fiber pathway: a review". Neuroscience. 98 (3): 407–427. doi:10.1016/s0306-4522(00)00146-9. PMID 10869836. S2CID 11663807.
- ↑ Scheibel P, et al. (1974). "The hippocampal-dentate complex in temporal lobe epilepsy". Epilepsia. 15 (1): 55–80. doi:10.1111/j.1528-1157.1974.tb03997.x. PMID 4523024. S2CID 35259630.
- ↑ Steward O (1992). "Lesion-induced synapse reorganization in the hippocampus of cats: sprouting of entorhinal, commissural/associational, and mossy fiber projections after unilateral entorhinal cortex lesions, with comments on the normal organization of these pathways". Hippocampus. 2 (3): 247–68. doi:10.1002/hipo.450020305. PMID 1284974. S2CID 24063369.
- ↑ Babb T. L.; et al. (1991). "Synaptic reorganization by mossy fibers in human epileptic fascia dentata". Neuroscience. 42 (2): 351–363. doi:10.1016/0306-4522(91)90380-7. PMID 1716744. S2CID 34237315.
- ↑ Buckmaster P, et al. (2002). "Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit". Journal of Neuroscience. 22 (15): 6650–6658. doi:10.1523/JNEUROSCI.22-15-06650.2002. PMC 6758164. PMID 12151544.
- ↑ Nadler J. V.; et al. (1985). "Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats". Journal of Neuroscience. 5 (4): 1016–1022. doi:10.1523/JNEUROSCI.05-04-01016.1985. PMC 6565006. PMID 3981241.
- ↑ Scharfman H. E.; et al. (2003). "Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting". Journal of Neurophysiology. 90 (4): 2536–2547. CiteSeerX 10.1.1.326.233. doi:10.1152/jn.00251.2003. PMID 14534276.
- ↑ Sloviter R. S.; et al. (2006). "Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyperinhibition in chronically epileptic rats". Journal of Comparative Neurology. 494 (6): 944–60. doi:10.1002/cne.20850. PMC 2597112. PMID 16385488.
- ↑ Sloviter R. S.; et al. (2003). "Dormant basket cell hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat". Journal of Comparative Neurology. 459 (1): 44–76. doi:10.1002/cne.10630. PMID 12629666. S2CID 36798455.
- ↑ Tu B, et al. (2005). "Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain". Journal of Neuroscience. 25 (7): 1718–1729. doi:10.1523/JNEUROSCI.4835-04.2005. PMC 6725947. PMID 15716408.
- ↑ Schwarzer C.; Sperk G. (1995). "Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat". Neuroscience. 69 (3): 705–709. doi:10.1016/0306-4522(95)00348-m. PMID 8596641. S2CID 2947533.
- ↑ "Temporal Lobe Epilepsy Workup: Approach Considerations, Computed Tomography Scanning, Magnetic Resonance Imaging". emedicine.medscape.com. Archived from the original on 23 August 2016. Retrieved 24 August 2016.
- 1 2 Duncan JS (October 2019). "Brain imaging in epilepsy". Practical Neurology. 19 (5): 438–443. doi:10.1136/practneurol-2018-002180. PMID 31420416. S2CID 201041577.
- ↑ "Temporal Lobe Epilepsy; TLE medical Information Page | Patient". Patient. Archived from the original on 29 August 2016. Retrieved 24 August 2016.
- ↑ Kwan P (2000). "Early identification of refractory epilepsy". NEJM. 342 (5): 314–319. doi:10.1056/NEJM200002033420503. PMID 10660394.
- ↑ Wiebe S, et al. (2001). "A randomized, controlled trial of surgery for temporal lobe epilepsy". NEJM. 345 (5): 311–318. doi:10.1056/NEJM200108023450501. PMID 11484687. S2CID 31539171.
- ↑ Cheung; et al. (2009). "Pre- and postoperative fMRI and clinical memory performance in temporal lobe epilepsy". J Neurol Neurosurg Psychiatry. 80 (10): 1099–1106. doi:10.1136/jnnp.2009.173161. hdl:10397/33373. PMID 19389718. S2CID 5602939.
- ↑ Maccotta L, et al. (2007). "Changing frontal contributions to memory before and after medial temporal lobectomy". Cereb Cortex. 17 (2): 443–456. doi:10.1093/cercor/bhj161. PMID 16547345.
- ↑ Helmstaedter C, et al. (2008). "The effects of cognitive rehabilitation on memory outcome after temporal lobe epilepsy surgery". Epilepsy Behav. 12 (3): 402–409. doi:10.1016/j.yebeh.2007.11.010. PMID 18155965. S2CID 29690584.
- ↑ Freeman, JM; Kossoff, EH; Hartman, AL (March 2007). "The ketogenic diet: one decade later". Pediatrics. 119 (3): 535–43. doi:10.1542/peds.2006-2447. PMID 17332207. S2CID 26629499. Archived from the original on 18 November 2022. Retrieved 17 November 2022.
- ↑ Curry, Daniel J.; Gowda, Ashok; McNichols, Roger J.; Wilfong, Angus A. (2012). "MR-guided stereotactic laser ablation of epileptogenic foci in children". Epilepsy & Behavior. 24 (4): 408–414. doi:10.1016/j.yebeh.2012.04.135. PMID 22687387. S2CID 531323. Archived from the original on 23 January 2021. Retrieved 17 November 2022.
- ↑ Ramachandran, V. and Blakeslee (1998). Phantoms in the Brain.
- 1 2 Craig Aaen-Stockdale (2012). "Neuroscience for the Soul". The Psychologist. 25 (7): 520–523. Archived from the original on 26 October 2015.
- ↑ Tedrus, Glória Maria Almeida Souza; Fonseca, Lineu Corrêa; Fagundes, Tatiane Mariani; da Silva, Gabriela Leopoldino (2015). "Religiosity aspects in patients with epilepsy". Epilepsy & Behavior. 50: 67–70. doi:10.1016/j.yebeh.2015.06.003. PMID 26133113. S2CID 22703938.
- ↑ Tedrus, Glória Maria Almeida Souza; Fonseca, Lineu Corrêa; Höehr, Gabriela Chaves (2013). "Spirituality aspects in patients with epilepsy". Seizure. 23 (1): 25–8. doi:10.1016/j.seizure.2013.09.005. PMID 24094727.
Patients with mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS) had significantly higher SSRS scores than those with other epileptic syndromes and, than in individuals of the CG (control Group)
- ↑ d'Orsi, Giuseppe; Tinuper, Paolo (2006). ""I heard voices...": from semiology, a historical review, and a new hypothesis on the presumed epilepsy of Joan of Arc". Epilepsy & Behavior. 9 (1): 152–157. doi:10.1016/j.yebeh.2006.04.020. PMID 16750938. S2CID 24961015.
- ↑ Murray E. D.; et al. (2012). "The role of psychotic disorders in religious history considered". The Journal of Neuropsychiatry and Clinical Neurosciences. 24 (4): 410–426. doi:10.1176/appi.neuropsych.11090214. PMID 23224447. S2CID 207654711. Archived from the original on 5 February 2022. Retrieved 17 November 2022.
- ↑ Sirven, Joseph I; Drazkowski, Joseph F; Noe, Katherine H (2007). "Seizures among public figures: lessons learned from the epilepsy of Pope Pius IX". Mayo Clinic Proceedings. 82 (12): 1535–1540. doi:10.1016/S0025-6196(11)61100-2. PMID 18053463.
- ↑ Arzy, Shahar; Schurr, Roey (2016). ""God has sent me to you": Right temporal epilepsy, left prefrontal psychosis". Epilepsy & Behavior. 60: 7–10. doi:10.1016/j.yebeh.2016.04.022. PMID 27176877. Lay summary.
In this patient, a messianic revelation experience occurred several hours after a complex partial seizure of temporal origin, compatible with postictal psychosis (PIP)
{{cite journal}}: Cite uses deprecated parameter|lay-url=(help) - ↑ Benson, D.F. & Hermann, B.P. (1998) Personality disorders. In J. Engel Jr. & T.A. Pedley (Eds.) Epilepsy: A comprehensive textbook. Vol. II (pp. 2065–2070). Philadelphia: Lippincott–Raven.
| Classification | |
|---|---|
| External resources |