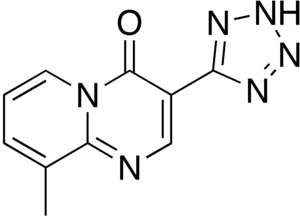
Pemirolast
 | |
| Names | |
|---|---|
| Trade names | Alamast, Alegysal, others |
| Other names | Pemirolast potassium |
IUPAC name
| |
| Clinical data | |
| Drug class | Mast cell stabilizer[1] |
| Main uses | Allergic conjunctivitis[1] |
| Side effects | Headache, runny nose[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, eye drop |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C10H8N6O |
| Molar mass | 228.215 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Pemirolast, sold under the brand names Alamast among others, is a medication used to treat allergic conjunctivitis.[1] It is used as an eye drop.[1]
Common side effects include headache and runny nose.[1] Safety in pregnancy and breastfeeding is unclear.[2] It is is a mast cell stabilizer.[1]
Pemirolast was approved for medical use in the United States in 1999.[1] As of 2021 it is not commercially available in the United States.[3]
Medical uses
The combination of levocabastine and pemirolast may be more effective than levocabastine alone.[5]
Dosage
It may be used as 1 to 2 drops up to 4 times per day.[1]
Research
It has also been studied for the treatment of asthma. Pemirolast has appeared as a possible candidate for SARS-CoV-2 (COVID-19) spike protein disruption and interference. Such results were ascertained by molecular dynamics calculations executed on the Summit supercomputer. By simulating compounds with FDA or similar regulatory approval, the authors found 4 interfacial molecules that could potentially disrupt the SARS-CoV-2 interface with ACE-2 receptors, suggesting that such small molecules could mitigate SARS-CoV-2 infection. The 4 candidate interfacial molecules included pemirolast, isoniazid pyruvate, nitrofurantoin, and eriodictyol.[6]
References
- 1 2 3 4 5 6 7 8 9 "Pemirolast Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 27 October 2021.
- 1 2 "Pemirolast ophthalmic (Alamast) Use During Pregnancy". Drugs.com. 2 September 2020. Archived from the original on 23 November 2020. Retrieved 13 September 2020.
- ↑ "Pemirolast Prices and Pemirolast Coupons - GoodRx". GoodRx. Archived from the original on 13 June 2016. Retrieved 27 October 2021.
- ↑ Mishra, Gyan P.; Tamboli, Viral; Jwala, Jwala; Mitra, Ashim K. (January 2011). "Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis". Recent Patents on Inflammation & Allergy Drug Discovery. 5 (1): 26–36. doi:10.2174/187221311794474883. ISSN 1872-213X.
- ↑ Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A (2015). "Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis". Cochrane Database Syst Rev. 6 (6): CD009566. doi:10.1002/14651858.CD009566.pub2. hdl:2164/6048. PMID 26028608.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Smith, MD, Smith JC (April 2020). "Repurposing Therapeutics for COVID-19: Supercomputer-Based Docking to the SARS-CoV-2 Viral Spike Protein and Viral Spike Protein-Human ACE2 Interface". Preprint: 1–28. Archived from the original on 2020-10-28. Retrieved 2020-12-12.
External links
| Identifiers: |
|---|
- "Pemirolast Potassium". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-21. Retrieved 2020-12-12.