Phosphofructokinase deficiency
| Phosphofructokinase deficiency | |
|---|---|
| Other names: Glycogen storage disease type VII or Tarui's disease[1][2] | |
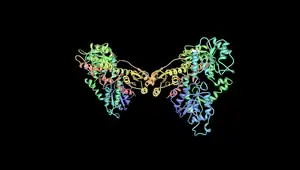 | |
| A rendering of the human muscular form of phosphofructokinase. Mutations in the production of this enzyme are the cause of Tarui's disease.[3] The symmetry of the enzyme is a result of its tetrameric structure. | |
Phosphofructokinase deficiency is a rare muscular metabolic disorder, with an autosomal recessive inheritance pattern.
It may affect humans as well as other mammals (especially dogs).[4] It was named after the Japanese physician Seiichiro Tarui (1927– ), who first observed the condition in 1965.[5]
Signs and symptoms
In humans
Human PFK deficiency is categorized into four types: classic, late-onset, infantile and hemolytic. These types are differentiated by age at which symptoms are observed and which symptoms present.[6]
Classic form
Classic phosphofructokinase deficiency is the most common type of this disorder. This type presents with exercise-induced muscle cramps and weakness (sometimes rhabdomyolysis), myoglobinuria, as well as with haemolytic anaemia causing dark urine a few hours later.[7] Hyperuricemia is common, due to the kidneys' inability to process uric acid following damage resulting from processing myoglobin. Nausea and vomiting following strenuous exercise is another common indicator of classic PFK deficiency. Many patients will also display high levels of bilirubin, which can lead to a jaundiced appearance. Symptoms for this type of PFK deficiency usually appear in early childhood.
Late-onset form
Late-onset PFK deficiency, as the name suggests, is a form of the disease that presents later in life. Common symptoms associated with late-onset phosphofructokinase deficiency are myopathy, weakness and fatigue. Many of the more severe symptoms found in the classic type of this disease are absent in the late-onset form.
Infantile form
Phosphofructokinase deficiency also presents in a rare infantile form. Infants with this deficiency often display floppy infant syndrome (hypotonia), arthrogryposis, encephalopathy and cardiomyopathy. The disorder can also manifest itself in the central nervous system, usually in the form of seizures. PFK deficient infants also often have some type of respiratory issue. Survival rate for the infantile form of PFK deficiency is low, and the cause of death is often due to respiratory failure.
Hemolytic form
The defining characteristic of this form of the disorder is hemolytic anemia, in which red blood cells break down prematurely. Muscle weakness and pain are not as common in patients with hemolytic PFK deficiency.
In dogs
Presentation of the canine form of the disease is similar to that of the human form. Most notably, PFK deficient dogs have mild, but persistent, anemia with hemolytic episodes, exercise intolerance, hemoglobinuria, and pale or jaundiced mucous membranes.[8] Muscle weakness and cramping are not uncommon symptoms, but they are not as common as they are in human PFKM deficiency.
Risk factors
In humans
In order to get Tarui's disease, both parents must be carriers of the genetic defect so that the child is born with the full form of the recessive trait. The best indicator of risk is a family member with PFK deficiency.[9]
In dogs
Canine phosphofructokinase deficiency is found mostly in English Springer Spaniels and American Cocker Spaniels, but has also been reported in Whippets and Wachtelhunds.[10][11] Mixed-breed dogs descended from any of these breeds are also at risk to inherit PFK deficiency.
Mechanism
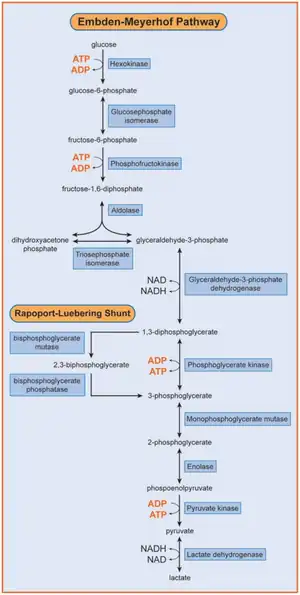
Phosphofructokinase is a tetrameric enzyme that consists of three types of subunits: PFKL (liver), PFKM (muscle), and PFKP (platelet). The combination of these subunits varies depending on the tissue in question.[12] In this condition, a deficiency of the M subunit (PFKM) of the phosphofructokinase enzyme impairs the ability of cells such as erythrocytes and rhabdomyocytes (skeletal muscle cells) to use carbohydrates (such as glucose) for energy. Unlike most other glycogen storage diseases, it directly affects glycolysis.[13] The mutation impairs the ability of phosphofructokinase to phosphorylate fructose-6-phosphate prior to its cleavage into glyceraldehyde-3-phosphate which is the rate limiting step in the glycolysis pathway. Inhibition of this step prevents the formation of adenosine triphosphate (ATP) from adenosine diphosphate (ADP), which results in a lack of available energy for muscles during heavy exercise. This results in the muscle cramping and pain that are common symptoms of the disease.[14]
In humans
Genetic mutation is the cause of phosphofructokinase deficiency. Several different mutations in the gene that encodes for PFKM have been reported in humans, but the result is production of PFKM subunits with little to no function.[15] As a result, affected individuals display only about 50–65% of total normal phosphofructokinase enzyme function.[16]
In dogs
PFK deficiency is believed to be the result of a nonsense mutation in the gene that encodes for PFKM. This results in an unstable, truncated protein that lacks normal function. This results in a near complete loss of PFKM activity in the skeletal muscle. Dogs with the mutation display 10–20% of normal PFK activity in their erythrocytes, due to a higher proportion of PFKM in those cells.[17]
Diagnosis

Symptoms of phosphofructokinase deficiency can closely resemble those of other metabolic diseases, include deficiencies of phosphoglycerate kinase, phosphoglycerate mutase, lactate dehydrogenase, beta-enolase and aldolase A.[7]
Thus, proper diagnosis is important to determine a treatment plan.
A diagnosis can be made through a muscle biopsy that shows excess glycogen accumulation. Glycogen deposits in the muscle are a result of the interruption of normal glucose breakdown that regulates the breakdown of glycogen. Blood tests are conducted to measure the activity of phosphofructokinase, which would be lower in a patient with this condition.[19] Patients also commonly display elevated levels of creatine kinase.[7]
Management
Treatment usually entails that the patient refrain from strenuous exercise to prevent muscle pain and cramping. Avoiding carbohydrates is also recommended.[20]
A ketogenic diet also improved the symptoms of an infant with PFK deficiency. The logic behind this treatment is that the low-carb high fat diet forces the body to use fatty acids as a primary energy source instead of glucose. This bypasses the enzymatic defect in glycolysis, lessening the impact of the mutated PFKM enzymes. This has not been widely studied enough to prove if it is a viable treatment, but testing is continuing to explore this option.[21]
Genetic testing to determine whether or not a person is a carrier of the mutated gene is also available.
In dogs
Diagnosis of canine phosphofructokinase deficiency is similar to the blood tests used in diagnosis of humans. Blood tests measuring the total erythrocyte PFK activity are used for definitive diagnosis in most cases.[22] DNA testing for presence of the condition is also available.[23]
Treatment mostly takes the form of supportive care. Owners are advised to keep their dogs out of stressful or exciting situations, avoid high temperature environments and strenuous exercise. It is also important for the owner to be alert for any signs of a hemolytic episode. Dogs carrying the mutated form of the gene should be removed from the breeding population, in order to reduce incidence of the condition.
References
- ↑ synd/3022 at Who Named It?
- ↑ Tarui S, OKuno G, Ikura Y, Tanaka T, Suda M, Nishikawa M (1965). "Phosphofructokinase Deficiency In Skeletal Muscle. A New Type Of Glycogenosis". Biochem. Biophys. Res. Commun. 19 (4): 517–523. doi:10.1016/0006-291X(65)90156-7. PMID 14339001.
- ↑ Kloos, M; Straeter, N (2014). "4OMT". Acta Crystallogr F. 70: 578–582. doi:10.2210/pdb4omt/pdb. Archived from the original on 2017-07-06. Retrieved 2022-10-08.
- ↑ Stedman, H.; Stedman, H; Rajpurohit, Y; Henthorn, PS; Wolfe, JH; Patterson, DF; Giger, U (1996). "Molecular Basis of Canine Muscle Type Phosphofructokinase Deficiency". Journal of Biological Chemistry. 271 (33): 20070–20074. doi:10.1074/jbc.271.33.20070. PMID 8702726.
- ↑ Wu, Pei-Ling; Yang, Yung-Ning; Tey, Shu-Leei; Yang, San-Nan; Lin, Chien-Seng (25 June 2015). "Infantile form of muscle phosphofructokinase deficiency in a premature neonate". Pediatrics International. 57 (4): 746–749. doi:10.1111/ped.12616. PMID 26108272. S2CID 13064914.
- ↑ "Glycogen Storage Disease Type VII". Genetics Home Reference. US National Library of Medicine. Archived from the original on 2020-09-18. Retrieved 2022-10-08.
- 1 2 3 Toscano A, Musumeci O (October 2007). "Tarui disease and distal glycogenoses: clinical and genetic update". Acta Myol. 26 (2): 105–107. PMC 2949577. PMID 18421897.
- ↑ "Phosphofructokinase (PFK) deficiency". Canine Inherited Disorders Database. University of Prince Edward Island. Archived from the original on 2016-04-13. Retrieved 2022-10-08.
- ↑ Kassir, Kari. "Glycogen Storage Diseases (Glycogenoses; GSD)". Mount Sinai Hospital. Archived from the original on 2016-03-06. Retrieved 2022-10-08.
- ↑ "Phosphofructokinase Deficiency (PFK)". PennGen Laboratories. University of Pennsylvania. Archived from the original on 2016-07-02. Retrieved 2022-10-08.
- ↑ Inal Gultekin, G; Raj, K; Lehman, S; Hillstrom, A; Giger, U (December 2012). "Missense mutation in PFKM associated with muscle-type phosphofructokinase deficiency in the Wachtelhund dog". Molecular and Cellular Probes. 26 (6): 243–247. doi:10.1016/j.mcp.2012.02.004. PMC 3485442. PMID 22446493.
- ↑ Musumeci, Olimpia; Bruno, Claudio; Mongini, Tiziana; Rodolico, Carmelo; Aguennouz, M'hammed; Barca, Emanuele; Amati, Angela; Cassandrini, Denise; Serlenga, Luigi; Vita, Giuseppe; Toscano, Antonio (2012). "Clinical features and new molecular findings in muscle phosphofructokinase deficiency (GSD type VII)". Neuromuscular Disorders. 22 (4): 325–330. doi:10.1016/j.nmd.2011.10.022. PMID 22133655. S2CID 20133199.
- ↑ Nakajima H, Raben N, Hamaguchi T, Yamasaki T (2002). "Phosphofructokinase deficiency; past, present and future". Curr. Mol. Med. 2 (2): 197–212. doi:10.2174/1566524024605734. PMID 11949936. Archived from the original on 2013-04-14. Retrieved 2022-10-08.
- ↑ Layzer, Robert; Rowland, Lewis; Ranney, Helen (November 1967). "Muscle Phosphofructokinase Deficiency". Journal of the American Medical Association. 17 (5): 512–523. doi:10.1001/archneur.1967.00470290066009. PMID 4228297.
- ↑ Raben, N; Sherman, JB (1995). "Mutations in muscle phosphofructokinase gene". Human Mutation. 6 (1): 1–6. doi:10.1002/humu.1380060102. PMID 7550225. S2CID 46649815. Archived from the original on 2022-12-04. Retrieved 2022-10-08.
- ↑ Vora, Shobhana; Giger, Urs; Turchen, Steven; Harvey, John (December 1985). "Characterization of the enzymatic lesion in inherited phosphofructokinase deficiency in the dog: an animal analogue of human glycogen storage disease type VII". Proceedings of the National Academy of Sciences of the United States of America. 82 (23): 8109–8113. Bibcode:1985PNAS...82.8109V. doi:10.1073/pnas.82.23.8109. PMC 391452. PMID 2933748.
- ↑ Harvey, J.W.; Smith, J.E. (June 1994). "Haematology and Clinical Chemistry of English Springer Spaniel Dogs with Phosphofructokinase Deficiency". Comparative Haematology International. 4 (2): 70–75. doi:10.1007/BF00368272. S2CID 25755199.
- ↑ Malfatti, Edoardo; Birouk, Nazha; Romero, Norma B.; Piraud, Monique; Petit, François M.; Hogrel, Jean-Yves; Laforêt, Pascal (2012-05-15). "Juvenile-onset permanent weakness in muscle phosphofructokinase deficiency". Journal of the Neurological Sciences. 316 (1–2): 173–177. doi:10.1016/j.jns.2012.01.027. PMID 22364848. S2CID 19654051.
- ↑ Ronquist, Gunnar. "Tarui disease". The Swedish Information Center for Rare Diseases. University of Gothenburg. Archived from the original on 2016-02-15. Retrieved 2022-10-08.
- ↑ "Glycogen Storage Disease Type VII". Rare Disease Database. National Organization for Rare Disorders. Archived from the original on 2016-05-03. Retrieved 2022-10-08.
- ↑ Swoboda, Kathryn; Specht, Linda; Jones, Royden; Shapiro, Frederic; DiMauro, Salvatore; Korson, Mark (December 1997). "Infantile phosphofructokinase deficiency with arthrogryposis: Clinical benefit of a ketogenic diet". The Journal of Pediatrics. 131 (6): 932–934. doi:10.1016/s0022-3476(97)70048-9. PMID 9427905.
- ↑ Gerber, Karen; Harvey, John; D'Agorne, Sara; Wood, Jonathan; Giger, Urs (March 2009). "Hemolysis, myopathy, and cardiac disease associated with hereditary phosphofructokinase deficiency in two Whippets". Veterinary Clinical Pathology. 38 (1): 46–51. doi:10.1111/j.1939-165X.2008.00089.x. PMC 2692053. PMID 19228357.
- ↑ Giger, U; Kimmel, A; Overlery, D; Schwartz, B; Smith, B; Rajpurohit, Y. "Frequency of Phosphofructokinase (PFK) Deficiency in English Springer Spaniels: A Longitudinal and Randomized Study". English Springer Spaniel Field Trial Association. Archived from the original on 2016-05-03. Retrieved 2022-10-08.
External links
- Glycogen storage disease type 7; Muscle phosphofructokinase deficiency; Tarui disease at NIH's Office of Rare Diseases
| Classification | |
|---|---|
| External resources |