Primary hyperoxaluria
| Primary hyperoxaluria | |
|---|---|
Primary hyperoxaluria is a rare condition (autosomal recessive), resulting in increased excretion of oxalate (up to 600 mg a day from normal 50 mg a day), with oxalate stones being common.
Signs and symptoms
Primary hyperoxaluria is an autosomal recessive disease, meaning both copies of the gene contain the mutation. Both parents must have one copy of this mutated gene to pass it on to their child, but they do not typically show signs or symptoms of the disease.
A single kidney stone in children or recurrent stones in adults is often the first warning sign of primary hyperoxaluria. Other symptoms range from recurrent urinary tract infections, severe abdominal pain or pain in the side, blood in the urine, to chronic kidney disease and kidney failure.[1] The age of symptom onset, progression and severity can vary greatly from one person to another, even among members of the same family. Some individuals may have mild cases that go undiagnosed well into adulthood; others may develop severe complications during infancy, which may result in early death.[2][3]
Pathophysiology

The buildup of oxalate in the body causes increased renal excretion of oxalate (hyperoxaluria), which in turn results in kidney and bladder stones. Stones cause urinary obstruction (often with severe and acute pain), secondary infection of urine and eventually kidney damage.[2] Primary hyperoxaluria is caused by genetic defects that result in the overproduction of oxalate. This is different from secondary hyperoxaluria, which is caused by the increase in dietary and intestinal absorption of oxalate or excessive intake of oxalate precursors.[4]
Oxalate stones in primary hyperoxaluria tend to be severe, resulting in relatively early kidney damage (in teenage years to early adulthood), which impairs the excretion of oxalate leading to a further acceleration in accumulation of oxalate in the body.
After the development of kidney failure patients may get deposits of oxalate in the bones, joints and bone marrow. Severe cases may develop haematological problems such as anaemia and thrombocytopaenia. The deposition of oxalate in the body is sometimes called "oxalosis" to be distinguished from "oxaluria" which refers to oxalate in the urine.
Diagnosis
A diagnosis of primary hyperoxaluria is suspected based on presenting patient characteristics such as kidney stones in infants or children, recurrent kidney stones in adults, or family history of hyperoxaluria. In these patients, stone analysis and urine analysis are recommended to rule out secondary causes of hyperoxaluria. A definitive diagnosis of primary hyperoxaluria requires genetic testing. This is performed using a gene panel covering known mutations for all three types of primary hyperoxaluria.[5][6]
Classification
The three main types of primary hyperoxaluria (PH1, PH2, and PH3) are each associated with mutations in specific genes involved in the metabolism of glyoxylate, the precursor of oxalate. These mutations result in decreased production or activity of the proteins that are involved in the normal breakdown of glyoxylate, which results in an overproduction of oxalate.[7] Mutations in the genes AGXT and GRHPR cause PH1 and PH2, respectively, through decreased production or activity of the proteins they make, which stops the normal breakdown of glyoxylate. Similarly, mutations in the gene HOGA1 cause PH3 due to loss-of-function mutations resulting in impaired protein function.[8]
PH1 is considered to be the most common and rapidly progressing form, accounting for about 80% of all currently diagnosed cases and PH2 and PH3 accounting for approximately 10% each of the current cases.[4][9] However, recent evidence has suggested that PH2 and PH3 are not as benign as previously thought, with up to 50% of patients with PH2 developing kidney failure (chronic kidney disease [CKD] stage 5).[9] While current estimates indicate that kidney failure is rarer in patients with PH3 compared to PH1 and PH2, CKD has been reported in patients with PH3. Moreover, the genetic prevalence based on known PH3 variants is much higher than the diagnosed prevalence of the disease, which could mean either incomplete penetrance (i.e. variant present with no clinical symptoms) or underdiagnosis (i.e. variant present with clinical symptoms but not diagnosed).[10]
| Type | OMIM | Gene |
|---|---|---|
| PH1 | 259900 | AGXT |
| PH2 | 260000 | GRHPR |
| PH3 | 613616 | HOGA1[11] |
Treatment
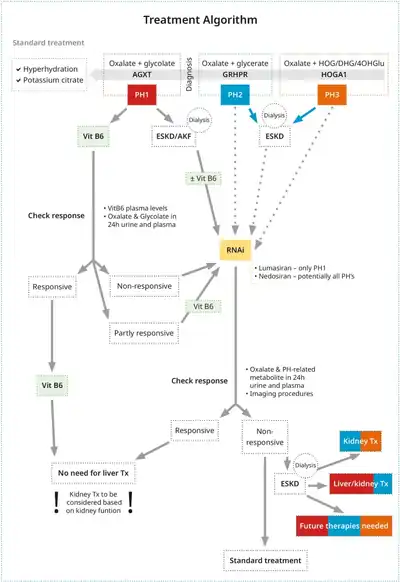
Increased water intake and alkalinization of urine is advised to prevent oxalate precipitation in urinary tract. In addition, Vitamin B6 (pyridoxine) is used to treat PH1 because alanine glyoxylate transaminase requires pyridoxine as cofactor. In approximately one third of patients with PH1, pyridoxine treatment decreases oxalate excretion and prevent kidney stone formation.[9] Conversely, a restriction in oxalate intake is of limited use as the main source of oxalate is endogenous in primary hyperoxaluria.[13]
Lumasiran, an RNA interference therapeutic drug,[14] is indicated for the treatment of primary hyperoxaluria type 1 (PH1) in adults and children of all ages and is available under the UK Early Access to Medicines Scheme (EAMS).[15] Lumasiran was approved for medical use in the European Union and in the United States in November 2020.[16][17] In addition, there are a few agents under investigation in clinical trials for PH: Nedosiran (RNA interference therapeutic) for PH1, PH2, and PH3; Stiripentol (antiepileptic drug); Oxabact (lyophilized Oxalobacter formigenes; and Reloxaliase (oxalate-digesting enzyme) for PH [18][19]
Treatment of renal failure in primary hyperoxaluria
Kidney failure is a serious complication requiring treatment in its own right. Dialysis can control kidney failure but tends to be inadequate to dispose of excess oxalate. Renal transplant is more effective and is the primary treatment of severe hyperoxaluria. Ultimately though, liver transplantation (often in addition to renal transplant) is required to correct the underlying metabolic defect.[20][21]
See also
References
- ↑ "LEARN Oxalosis & Hyperoxaluria | Oxalosis & Hyperoxaluria Foundation". www.ohf.org. Archived from the original on 2021-10-28. Retrieved 2023-09-14.
- 1 2 "Primary Hyperoxaluria". NORD (National Organization for Rare Disorders). Archived from the original on 2021-12-27. Retrieved 2023-09-14.
- ↑ Hopp, Katharina; Cogal, Andrea G.; Bergstralh, Eric J.; Seide, Barbara M.; Olson, Julie B.; Meek, Alicia M.; Lieske, John C.; Milliner, Dawn S.; Harris, Peter C. (1 October 2015). "Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria". Journal of the American Society of Nephrology. 26 (10): 2559–2570. doi:10.1681/ASN.2014070698. ISSN 1046-6673. PMC 4587693. PMID 25644115.
- 1 2 Bhasin, Bhavna; Ürekli, Hatice Melda; Atta, Mohamed G (6 May 2015). "Primary and secondary hyperoxaluria: Understanding the enigma". World Journal of Nephrology. 4 (2): 235–244. doi:10.5527/wjn.v4.i2.235. ISSN 2220-6124. PMC 4419133. PMID 25949937.
- ↑ "Test ID: HYOX Hyperoxaluria Panel, Random, Urine" (PDF). Mayo clinic laboratories. Archived (PDF) from the original on 13 June 2021. Retrieved 14 April 2021.
- ↑ Edvardsson, Vidar O.; Goldfarb, David S.; Lieske, John C.; Beara-Lasic, Lada; Anglani, Franca; Milliner, Dawn S.; Palsson, Runolfur (October 2013). "Hereditary Causes of Kidney Stones and Chronic Kidney Disease". Pediatric Nephrology (Berlin, Germany). 28 (10): 1923–1942. doi:10.1007/s00467-012-2329-z. ISSN 0931-041X. PMC 4138059. PMID 23334384.
- ↑ Hulton, SA (December 2016). "The primary hyperoxalurias: A practical approach to diagnosis and treatment". International Journal of Surgery (London, England). 36 (Pt D): 649–654. doi:10.1016/j.ijsu.2016.10.039. PMID 27815184.
- ↑ Milliner, DS; Harris, PC; Lieske, JC (September 24, 2015). "Primary Hyperoxaluria Type 3". In Adam MP; Ardinger HH; Pagon RA; et al. (eds.). GeneReviews. University of Washington, Seattle. PMID 26401545. Archived from the original on June 3, 2023. Retrieved September 14, 2023.
- 1 2 3 Weigert, Alexander; Martin-Higueras, Christina; Hoppe, Bernd (2018-10-02). "Novel therapeutic approaches in primary hyperoxaluria". Expert Opinion on Emerging Drugs. 23 (4): 349–357. doi:10.1080/14728214.2018.1552940. PMID 30540923. S2CID 56149313.
- ↑ Forbes, TA; Brown, BD; Lai, C (22 May 2021). "Therapeutic RNA interference: A novel approach to the treatment of primary hyperoxaluria". British Journal of Clinical Pharmacology. 88 (6): 2525–2538. doi:10.1111/bcp.14925. PMC 9291495. PMID 34022071. S2CID 235126922.
- ↑ Belostotsky R, Seboun E, Idelson GH, et al. (September 2010). "Mutations in DHDPSL are responsible for primary hyperoxaluria type III". Am. J. Hum. Genet. 87 (3): 392–9. doi:10.1016/j.ajhg.2010.07.023. PMC 2933339. PMID 20797690.
- ↑ Hoppe, Bernd; Martin-Higueras, Cristina (July 2022). "Improving Treatment Options for Primary Hyperoxaluria". Drugs. 82 (10): 1077–1094. doi:10.1007/s40265-022-01735-x. ISSN 1179-1950. Retrieved 26 September 2023.
- ↑ https://iris.unito.it/retrieve/handle/2318/104759/16020/2012_Cochat_post-print.pdf Archived 2023-07-16 at the Wayback Machine
- ↑ Forbes, Thomas A.; Brown, Bob D.; Lai, Chengjung (2021-06-11). "Therapeutic RNA interference: A novel approach to the treatment of primary hyperoxaluria". British Journal of Clinical Pharmacology. 88 (6): 2525–2538. doi:10.1111/bcp.14925. PMC 9291495. PMID 34022071. S2CID 235126922.
- ↑ "Lumasiran: Public Assessment Report (PAR)" (PDF). Medicines and Healthcare products Regulatory Agency (MHRA). Archived (PDF) from the original on 21 October 2020. Retrieved 17 October 2020. Contains public sector information licensed under the Open Government Licence v3.0.
- ↑ "Oxlumo EPAR". European Medicines Agency (EMA). 13 October 2020. Archived from the original on 10 January 2021. Retrieved 26 December 2020.
- ↑ "FDA Approves First Drug to Treat Rare Metabolic Disorder". U.S. Food and Drug Administration (FDA) (Press release). 23 November 2020. Archived from the original on 23 November 2020. Retrieved 23 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Shah, Aniruddh; Leslie, Stephen W.; Ramakrishnan, Sharanya (2022). "Hyperoxaluria". StatPearls. StatPearls Publishing. PMID 32644413. Archived from the original on 2022-12-19. Retrieved 2023-09-14.
- ↑ Shee, K; Stoller, ML (8 December 2021). "Perspectives in primary hyperoxaluria - historical, current and future clinical interventions". Nature Reviews. Urology. 19 (3): 137–146. doi:10.1038/s41585-021-00543-4. PMC 8652378. PMID 34880452.
- ↑ Lawrence, Jennifer E.; Wattenberg, Debra J. (12 March 2020). "Primary Hyperoxaluria: The Patient and Caregiver Perspective". Clinical Journal of the American Society of Nephrology. 15 (7): 909–911. doi:10.2215/CJN.13831119. ISSN 1555-9041. PMC 7341774. PMID 32165441. Archived from the original on 20 November 2021. Retrieved 14 September 2023.
- ↑ Milliner, Dawn S.; McGregor, Tracy L.; Thompson, Aliza; Dehmel, Bastian; Knight, John; Rosskamp, Ralf; Blank, Melanie; Yang, Sixun; Fargue, Sonia; Rumsby, Gill; Groothoff, Jaap; Allain, Meaghan; West, Melissa; Hollander, Kim; Lowther, W. Todd; Lieske, John C. (1 July 2020). "End Points for Clinical Trials in Primary Hyperoxaluria". Clinical Journal of the American Society of Nephrology. 15 (7): 1056–1065. doi:10.2215/CJN.13821119. ISSN 1555-9041. PMC 7341772. PMID 32165440. Archived from the original on 19 November 2021. Retrieved 14 September 2023.
External links
| Classification |
|---|
- GeneReview/NCBI/NIH/UW entry on Primary Hyperoxaluria Type 1 Archived 2010-06-10 at the Wayback Machine
- GeneReview/NCBI/NIH/UW entry on Primary Hyperoxaluria Type 2 Archived 2010-06-10 at the Wayback Machine
- Primary hyperoxaluria (A service of the U.S. National Library of Medicine) Archived 2010-04-08 at the Wayback Machine