Subthalamic nucleus
| Subthalamic nucleus | |
|---|---|
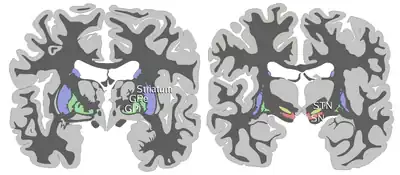 Coronal slices of human brain showing the basal ganglia (external globus pallidus (GPe) and internal globus pallidus (GPi)), subthalamic nucleus (STN) and substantia nigra (SN). | |
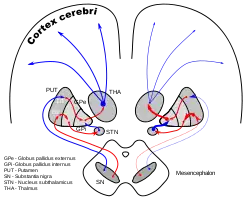 DA-loops in Parkinson's disease | |
| Details | |
| Part of | Basal ganglia |
| Identifiers | |
| Latin | nucleus subthalamicus |
| MeSH | D020531 |
| NeuroNames | 435 |
| NeuroLex ID | nlx_anat_1010002 |
| TA98 | A14.1.08.702 |
| TA2 | 5709 |
| FMA | 62035 |
| Anatomical terms of neuroanatomy | |
The subthalamic nucleus is a small lens-shaped nucleus in the brain where it is, from a functional point of view, part of the basal ganglia system. In terms of anatomy, it is the major part of the subthalamus. As suggested by its name, the subthalamic nucleus is located ventral to the thalamus. It is also dorsal to the substantia nigra and medial to the internal capsule. It was first described by Jules Bernard Luys in 1865,[1] and the term corpus Luysi or Luys' body is still sometimes used.
Anatomy
Structure
The principal type of neuron found in the subthalamic nucleus has rather long, sparsely spiny dendrites.[2][3] In the more centrally located neurons, the dendritic arbors have a more ellipsoidal shape.[4] The dimensions of these arbors (1200 μm, 600 μm, and 300 μm) are similar across many species—including rat, cat, monkey and human—which is unusual. However, the number of neurons increases with brain size as well as the external dimensions of the nucleus. The principal neurons are glutamatergic, which give them a particular functional position in the basal ganglia system. In humans there are also a small number (about 7.5%) of GABAergic interneurons that participate in the local circuitry; however, the dendritic arbors of subthalamic neurons shy away from the border and primarily interact with one another.[5]
Afferent axons
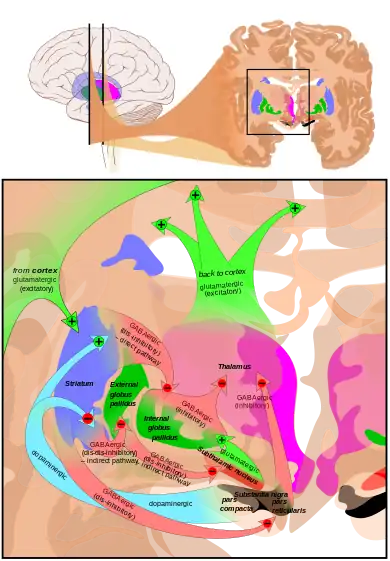
The subthalamic nucleus receives its main input from the external globus pallidus (GPe),[6] not so much through the ansa lenticularis as often said but by radiating fibers crossing the medial pallidum first and the internal capsule (see figure). These afferents are GABAergic, inhibiting neurons in the subthalamic nucleus. Excitatory, glutamatergic inputs come from the cerebral cortex (particularly the motor cortex), and from the pars parafascicularis of the central complex. The subthalamic nucleus also receives neuromodulatory inputs, notably dopaminergic axons from the substantia nigra pars compacta.[7] It also receives inputs from the pedunculopontine nucleus.
Efferent targets
The axons of subthalamic nucleus neurons leave the nucleus dorsally. The efferent axons are glutamatergic (excitatory). Except for the connection to the striatum (17.3% in macaques), most of the subthalamic principal neurons are multitargets and directed to the other elements of the core of the basal ganglia.[8] Some send axons to the substantia nigra medially and to the medial and lateral nuclei of the pallidum laterally (3-target, 21.3%). Some are 2-target with the lateral pallidum and the substantia nigra (2.7%) or the lateral pallidum and the medial (48%). Less are single target for the lateral pallidum. In the pallidum, subthalamic terminals end in bands parallel to the pallidal border.[8][9] When all axons reaching this target are added, the main efference of the subthalamic nucleus is, in 82.7% of the cases, clearly the internal globus pallidus (GPi).
Some researchers have reported internal axon collaterals.[10] However, there is little functional evidence for this.
Physiology
Subthalamic nucleus
The first intracellular electrical recordings of subthalamic neurons were performed using sharp electrodes in a rat slice preparation. In these recordings three key observations were made, all three of which have dominated subsequent reports of subthalamic firing properties. The first observation was that, in the absence of current injection or synaptic stimulation, the majority of cells were spontaneously firing. The second observation is that these cells are capable of transiently firing at very high frequencies. The third observation concerns non-linear behaviors when cells are transiently depolarized after being hyperpolarized below –65mV. They are then able to engage voltage-gated calcium and sodium currents to fire bursts of action potentials.
Several recent studies have focused on the autonomous pacemaking ability of subthalamic neurons. These cells are often referred to as "fast-spiking pacemakers",[11] since they can generate spontaneous action potentials at rates of 80 to 90 Hz in primates.
Oscillatory and synchronous activity[12][13] is likely to be a typical pattern of discharge in subthalamic neurons recorded from patients and animal models characterized by the loss of dopaminergic cells in the substantia nigra pars compacta, which is the principal pathology that underlies Parkinson's disease.
Lateropallido-subthalamic system
Strong reciprocal connections link the subthalamic nucleus and the external segment of the globus pallidus. Both are fast-spiking pacemakers. Together, they are thought to constitute the "central pacemaker of the basal ganglia"[14] with synchronous bursts.
The connection of the lateral pallidum with the subthalamic nucleus is also the one in the basal ganglia system where the reduction between emitter/receiving elements is likely the strongest. In terms of volume, in humans, the lateral pallidum measures 808 mm³, the subthalamic nucleus only 158 mm³.[15] This translated in numbers of neurons represents a strong compression with loss of map precision.
Some axons from the lateral pallidum go to the striatum.[16] The activity of the medial pallidum is influenced by afferences from the lateral pallidum and from the subthalamic nucleus.[17] The same for the substantia nigra pars reticulata.[9] The subthalamic nucleus sends axons to another regulator: the pedunculo-pontine complex (id).
The lateropallido-subthalamic system is thought to play a key role in the generation of the patterns of activity seen in Parkinson's disease.[18]
Pathophysiology
Chronic stimulation of the STN, called deep brain stimulation (DBS), is used to treat patients with Parkinson disease. The first to be stimulated are the terminal arborisations of afferent axons, which modify the activity of subthalamic neurons. However, it has been shown in thalamic slices from mice,[19] that the stimulus also causes nearby astrocytes to release adenosine triphosphate (ATP), a precursor to adenosine (through a catabolic process). In turn, adenosine A1 receptor activation depresses excitatory transmission in the thalamus, thus mimicking ablation of the subthalamic nucleus.
Unilateral destruction or disruption of the subthalamic nucleus — which can commonly occur via a small vessel stroke in patients with diabetes, hypertension, or a history of smoking – produces hemiballismus.
As one of the STN's suspected functions is in impulse control, dysfunction in this region has been implicated in obsessive–compulsive disorder.[20] Artificially stimulating the STN has shown some promise in correcting severe impulsive behavior and may later be used as an alternative treatment for the disorder.[21]
Function
The function of the STN is unknown, but current theories place it as a component of the basal ganglia control system that may perform action selection. It is thought to implement the so-called "hyperdirect pathway" of motor control, contrasting with the direct and indirect pathways implemented elsewhere in the basal ganglia. STN dysfunction has also been shown to increase impulsivity in individuals presented with two equally rewarding stimuli.[22]
Research has suggested that the subthalamus is an extrapyramidal center. It holds muscular responses in check, and damage may result in hemiballism (a violent flinging of the arm and leg on one side of the body).[23]
The physiological role of the STN has been for long hidden by its pathological role. But lately, the research on the physiology of the STN lead to the discovery that the STN is required to achieve intended movement, including locomotion, balance and motor coordination. It is indeed involved in stopping or interrupting on-going motor tasks. Moreover, STN excitation was generally correlated with significant reduction in locomotor activity, while in contrast, STN inhibition enhanced locomotion.[24][25][26]
Additional images
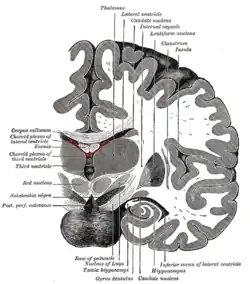 Coronal section of brain immediately in front of pons. Subthalamic nucleus labeled as "Nucleus of Luys".
Coronal section of brain immediately in front of pons. Subthalamic nucleus labeled as "Nucleus of Luys".
See also
| Wikimedia Commons has media related to Subthalamic nucleus. |
References
- ↑ Luys JB (1865). Recherches sur le système cérébro-spinal, sa structure, ses fonctions et ses maladies (in French). Paris: Baillière.
- ↑ Afsharpour S (June 1985). "Light microscopic analysis of Golgi-impregnated rat subthalamic neurons". The Journal of Comparative Neurology. 236 (1): 1–13. doi:10.1002/cne.902360102. PMID 4056088.
- ↑ Rafols JA, Fox CA (July 1976). "The neurons in the primate subthalamic nucleus: a Golgi and electron microscopic study". The Journal of Comparative Neurology. 168 (1): 75–111. doi:10.1002/cne.901680105. PMID 819471.
- ↑ Yelnik J, Percheron G (1979). "Subthalamic neurons in primates: a quantitative and comparative analysis". Neuroscience. 4 (11): 1717–43. doi:10.1016/0306-4522(79)90030-7. PMID 117397.
- ↑ Lévesque JC, Parent A (May 2005). "GABAergic interneurons in human subthalamic nucleus". Movement Disorders. 20 (5): 574–84. doi:10.1002/mds.20374. PMID 15645534.
- ↑ Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA (April 1990). "Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat". Brain Research. 513 (1): 43–59. doi:10.1016/0006-8993(90)91087-W. PMID 2350684.
- ↑ Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD (October 2004). "Synaptic release of dopamine in the subthalamic nucleus". The European Journal of Neuroscience. 20 (7): 1788–802. doi:10.1111/j.1460-9568.2004.03629.x. PMID 15380000.
- 1 2 Nauta HJ, Cole M (July 1978). "Efferent projections of the subthalamic nucleus: an autoradiographic study in monkey and cat". The Journal of Comparative Neurology. 180 (1): 1–16. doi:10.1002/cne.901800102. PMID 418083.
- 1 2 Smith Y, Hazrati LN, Parent A (April 1990). "Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method". The Journal of Comparative Neurology. 294 (2): 306–23. doi:10.1002/cne.902940213. PMID 2332533.
- ↑ Kita H, Chang HT, Kitai ST (April 1983). "The morphology of intracellularly labeled rat subthalamic neurons: a light microscopic analysis". The Journal of Comparative Neurology. 215 (3): 245–57. doi:10.1002/cne.902150302. PMID 6304154.
- ↑ Surmeier DJ, Mercer JN, Chan CS (June 2005). "Autonomous pacemakers in the basal ganglia: who needs excitatory synapses anyway?". Current Opinion in Neurobiology. 15 (3): 312–8. doi:10.1016/j.conb.2005.05.007. PMID 15916893.
- ↑ Levy R, Hutchison WD, Lozano AM, Dostrovsky JO (October 2000). "High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor". The Journal of Neuroscience. 20 (20): 7766–75. doi:10.1523/JNEUROSCI.20-20-07766.2000. PMC 6772896. PMID 11027240.
- ↑ Lintas A, Silkis IG, Albéri L, Villa AE (January 2012). "Dopamine deficiency increases synchronized activity in the rat subthalamic nucleus" (PDF). Brain Research. 1434 (3): 142–51. doi:10.1016/j.brainres.2011.09.005. PMID 21959175.
- ↑ Plenz D, Kital ST (August 1999). "A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus". Nature. 400 (6745): 677–82. doi:10.1038/23281. PMID 10458164.
- ↑ Yelnik J (2002). "Functional anatomy of the basal ganglia". Movement Disorders. 17 Suppl 3 (Suppl. 3): S15-21. doi:10.1002/mds.10138. PMID 11948751.
- ↑ Sato F, Lavallée P, Lévesque M, Parent A (January 2000). "Single-axon tracing study of neurons of the external segment of the globus pallidus in primate". The Journal of Comparative Neurology. 417 (1): 17–31. doi:10.1002/(SICI)1096-9861(20000131)417:1<17::AID-CNE2>3.0.CO;2-I. PMID 10660885.
- ↑ Smith Y, Wichmann T, DeLong MR (May 1994). "Synaptic innervation of neurones in the internal pallidal segment by the subthalamic nucleus and the external pallidum in monkeys". The Journal of Comparative Neurology. 343 (2): 297–318. doi:10.1002/cne.903430209. PMID 8027445.
- ↑ Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ (October 2002). "Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network". Trends in Neurosciences. 25 (10): 525–31. doi:10.1016/S0166-2236(02)02235-X. PMID 12220881.
- ↑ Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, et al. (January 2008). "Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor". Nature Medicine. 14 (1): 75–80. doi:10.1038/nm1693. PMID 18157140.
- ↑ Carter R. The Human Brain Book. pp. 58, 233.
- ↑ Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. (November 2008). "Subthalamic nucleus stimulation in severe obsessive-compulsive disorder". The New England Journal of Medicine. 359 (20): 2121–34. doi:10.1056/NEJMoa0708514. PMID 19005196.
- ↑ Frank MJ, Samanta J, Moustafa AA, Sherman SJ (November 2007). "Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism". Science. 318 (5854): 1309–12. doi:10.1126/science.1146157. PMID 17962524.
- ↑ Bruce H. Robinson (2007). Biomedicine - A textbook for Practitioners of Acupuncture & Oriental Medicine. Blue Poppy Press. p. 126. ISBN 978-1-891845-38-3. LCCN 2006940894.
- ↑ Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (April 2007). "Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI". The Journal of Neuroscience. 27 (14): 3743–52. doi:10.1523/JNEUROSCI.0519-07.2007. PMC 6672420. PMID 17409238.
- ↑ Fife KH, Gutierrez-Reed NA, Zell V, Bailly J, Lewis CM, Aron AR, Hnasko TS (July 2017). Uchida N (ed.). "Causal role for the subthalamic nucleus in interrupting behavior". eLife. 6: e27689. doi:10.7554/eLife.27689. PMC 5526663. PMID 28742497.
- ↑ Guillaumin A, Serra GP, Georges F, Wallén-Mackenzie Å (March 2021). "Experimental investigation into the role of the subthalamic nucleus (STN) in motor control using optogenetics in mice". Brain Research. 1755: 147226. doi:10.1016/j.brainres.2020.147226. PMID 33358727.