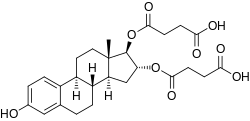
Estriol succinate
Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms.[1] It is taken by mouth, in through the vagina, and by injection.[1][2][3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Synapause, others |
| Other names | Oestriol succinate; Estriol disuccinate; Estriol hemisuccinate; Succinylestriol; Estriol 16α,17β-di(hydrogen succinate) |
| Routes of administration | By mouth, vaginal[1] |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ECHA InfoCard | 100.007.442 |
| Chemical and physical data | |
| Formula | C26H32O9 |
| Molar mass | 488.533 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medical uses
Estriol succinate is used in menopausal hormone therapy in the treatment and prevention of menopausal symptoms such as hot flashes, vaginal atrophy, and osteoporosis.[1] Unlike other estrogens, depending on how it is used (i.e., how often it is taken and at what dosage), estriol succinate may not require concomitant therapy with a progestogen to prevent endometrial hyperplasia and endometrial cancer in women with intact uteruses.[1]
The clinical effects of estriol succinate in the treatment of menopausal symptoms have been characterized in a large 5-year clinical trial of 911 menopausal women.[4][5][6]
Side effects
Pharmacology
Estriol succinate is an estrogen ester, specifically, an ester of estriol, and acts as a prodrug of estriol in the body.[7][1] It is described as a weak estrogen in comparison to estradiol valerate.[1][8] Estriol succinate is used medically via oral and vaginal routes similarly.[1] In estriol succinate, two of the hydroxyl groups of estriol, those at the C16α and C17β positions, are esterified with succinic acid.[1] As such, when adjusted for differences in molecular weight, a dose of 2 mg estriol succinate is equivalent to 1.18 mg unconjugated estriol.[1] Unlike other estrogen esters, such as estradiol valerate, estriol succinate is hydrolyzed almost not at all in the intestinal mucosa when taken orally, and in relation to this, is absorbed more slowly than is estriol.[1] Consequently, oral estriol succinate is a longer-acting form of estriol than oral estriol.[9] Instead of in the gastrointestinal tract, oral estriol succinate is cleaved into estriol mainly in the liver.[1] After a single 8 mg oral dose of estriol succinate, maximum levels of circulating estriol of 40 pg/mL are attained within 12 hours, and this increases up to 80 pg/mL with continued daily administration.[1]
| Compound | Dosage for specific uses (mg usually)[lower-alpha 1] | ||||||
|---|---|---|---|---|---|---|---|
| ETD[lower-alpha 2] | EPD[lower-alpha 2] | MSD[lower-alpha 2] | MSD[lower-alpha 3] | OID[lower-alpha 3] | TSD[lower-alpha 3] | ||
| Estradiol (non-micron.) | 30 | ≥120–300 | 120 | 6 | - | - | |
| Estradiol (micronized) | 6–12 | 60–80 | 14–42 | 1–2 | >5 | >8 | |
| Estradiol valerate | 6–12 | 60–80 | 14–42 | 1–2 | - | >8 | |
| Estradiol benzoate | - | 60–140 | - | - | - | - | |
| Estriol | ≥20 | 120–150[lower-alpha 4] | 28–126 | 1–6 | >5 | - | |
| Estriol succinate | - | 140–150[lower-alpha 4] | 28–126 | 2–6 | - | - | |
| Estrone sulfate | 12 | 60 | 42 | 2 | - | - | |
| Conjugated estrogens | 5–12 | 60–80 | 8.4–25 | 0.625–1.25 | >3.75 | 7.5 | |
| Ethinylestradiol | 200 μg | 1–2 | 280 μg | 20–40 μg | 100 μg | 100 μg | |
| Mestranol | 300 μg | 1.5–3.0 | 300–600 μg | 25–30 μg | >80 μg | - | |
| Quinestrol | 300 μg | 2–4 | 500 μg | 25–50 μg | - | - | |
| Methylestradiol | - | 2 | - | - | - | - | |
| Diethylstilbestrol | 2.5 | 20–30 | 11 | 0.5–2.0 | >5 | 3 | |
| DES dipropionate | - | 15–30 | - | - | - | - | |
| Dienestrol | 5 | 30–40 | 42 | 0.5–4.0 | - | - | |
| Dienestrol diacetate | 3–5 | 30–60 | - | - | - | - | |
| Hexestrol | - | 70–110 | - | - | - | - | |
| Chlorotrianisene | - | >100 | - | - | >48 | - | |
| Methallenestril | - | 400 | - | - | - | - | |
Chemistry
Estriol succinate, also known as estriol disuccinate or as estriol 16α,17β-di(hydrogen succinate), is a synthetic estrane steroid and a derivative of estriol.[7][29][30] It is specifically the C16α and C17β disuccinate ester of estriol.[7][29][30][1] The medication is provided both as estriol succinate and as estriol sodium succinate, the sodium salt.[7][29] Other marketed estriol esters besides estriol succinate include estriol acetate benzoate and estriol tripropionate, whereas estriol dihexanoate, estriol dipropionate, and estriol triacetate are estriol esters that were never marketed.[7][29] Quinestradol is an estriol ether and has also been marketed.[7][29] Polyestriol phosphate is an ester of estriol in the form of a polymer, and was previously marketed.[31][32][33][34]
History
Estriol succinate was introduced for medical use in 1966.[35]
Society and culture
Generic names
Estriol succinate is the generic name of the drug and its INNTooltip International Nonproprietary Name and BANTooltip British Approved Name.[7][29][36][30][35] Other synonyms include oestriol succinate, estriol disuccinate, and estriol hemisuccinate.[7][29][36][30] When provided as the sodium salt, estriol succinate is known as estriol sodium succinate (BANTooltip British Approved Name) or as oestriol sodium succinate.[7][29]
Brand names
Estriol succinate has been marketed under brand names including Blissel, Evalon, Gelistrol, Hemostyptanon, Orgastyptin, Ovestin, Sinapause, Styptanon, Synapsa, Synapasa, Synapausa, and Synapause, among others.[7][29][36][30] Estriol sodium succinate has been marketed specifically under the brand names Pausan and Styptanon.[7][29]
Research
Estriol succinate was under development for the treatment of multiple sclerosis in the United States and worldwide, and reached phase II clinical trials for this indication, but development was discontinued due to insufficient effectiveness.[37] It had the tentative brand name Trimesta.[37]
References
- Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Satoskar RS, Bhandarkar SD, Rege NN (1973). "Gonadotropins, Estrogens, and Progestins". Pharmacology and Pharmacotherapeutics. Popular Prakashan. pp. 934–. ISBN 978-81-7991-527-1.
- Kleemann A, Engel J (2001). Pharmaceutical substances: syntheses, patents, applications. Thieme. p. 786. ISBN 978-3-13-558404-1.
- Lauritzen C (November 1987). "Results of a 5 years prospective study of estriol succinate treatment in patients with climacteric complaints". Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et Metabolisme. 19 (11): 579–584. doi:10.1055/s-2007-1011886. PMID 3428874. S2CID 10551484.
- Ali ES, Mangold C, Peiris AN (September 2017). "Estriol: emerging clinical benefits". Menopause. 24 (9): 1081–1085. doi:10.1097/GME.0000000000000855. PMID 28375935. S2CID 41137736.
- Lommen E, Mead JH (2013). "Estriol; the 'Good' Estrogen Advances and Updates in its Clinical Uses". Journal of Restorative Medicine. 2 (1): 45–52. doi:10.14200/jrm.2013.2.0103. ISSN 2165-7971.
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 899. ISBN 978-1-4757-2085-3.
- Cutler WB, García CR (1984). The medical management of menopause and premenopause: their endocrinologic basis. Lippincott Williams & Wilkins. p. 31. ISBN 978-0-397-50631-6.
- Clark JH, Markaverich BM (1983). "The agonistic and antagonistic effects of short acting estrogens: a review". Pharmacology & Therapeutics. 21 (3): 429–453. doi:10.1016/0163-7258(83)90063-3. PMID 6356176.
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Lauritzen C (June 1977). "[Estrogen thearpy in practice. 3. Estrogen preparations and combination preparations]" [Estrogen therapy in practice. 3. Estrogen preparations and combination preparations]. Fortschritte Der Medizin (in German). 95 (21): 1388–92. PMID 559617.
- Wolf AS, Schneider HP (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- Göretzlehner G, Lauritzen C, Römer T, Rossmanith W (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4.
- Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598–599. ISBN 978-3-11-150424-7.
- Lauritzen CH (January 1976). "The female climacteric syndrome: significance, problems, treatment". Acta Obstetricia Et Gynecologica Scandinavica. Supplement. 51: 47–61. doi:10.3109/00016347509156433. PMID 779393.
- Lauritzen C (1975). "The Female Climacteric Syndrome: Significance, Problems, Treatment". Acta Obstetricia et Gynecologica Scandinavica. 54 (s51): 48–61. doi:10.3109/00016347509156433. ISSN 0001-6349.
- Kopera H (1991). "Hormone der Gonaden". Hormonelle Therapie für die Frau. Kliniktaschenbücher. pp. 59–124. doi:10.1007/978-3-642-95670-6_6. ISBN 978-3-540-54554-5. ISSN 0172-777X.
- Scott WW, Menon M, Walsh PC (April 1980). "Hormonal Therapy of Prostatic Cancer". Cancer. 45 (Suppl 7): 1929–1936. doi:10.1002/cncr.1980.45.s7.1929. PMID 29603164.
- Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgender Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- Ryden AB (1950). "Natural and synthetic oestrogenic substances; their relative effectiveness when administered orally". Acta Endocrinologica. 4 (2): 121–39. doi:10.1530/acta.0.0040121. PMID 15432047.
- Ryden AB (1951). "The effectiveness of natural and synthetic oestrogenic substances in women". Acta Endocrinologica. 8 (2): 175–91. doi:10.1530/acta.0.0080175. PMID 14902290.
- Kottmeier HL (1947). "Ueber blutungen in der menopause: Speziell der klinischen bedeutung eines endometriums mit zeichen hormonaler beeinflussung: Part I". Acta Obstetricia et Gynecologica Scandinavica. 27 (s6): 1–121. doi:10.3109/00016344709154486. ISSN 0001-6349.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 oestradiol or with 380 oestrone.
- Rietbrock N, Staib AH, Loew D (11 March 2013). Klinische Pharmakologie: Arzneitherapie. Springer-Verlag. pp. 426–. ISBN 978-3-642-57636-2.
- Martinez-Manautou J, Rudel HW (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- Herr F, Revesz C, Manson AJ, Jewell JB (1970). "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. pp. 368–408. doi:10.1007/978-3-642-49793-3_8. ISBN 978-3-642-49506-9.
- Duncan CJ, Kistner RW, Mansell H (October 1956). "Suppression of ovulation by trip-anisyl chloroethylene (TACE)". Obstetrics and Gynecology. 8 (4): 399–407. PMID 13370006.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 407–. ISBN 978-3-88763-075-1.
- "Estriol".
- Lauritzen C, Velibese S (September 1961). "Clinical investigations of a long-acting oestriol (polyoestriol phosphate)". Acta Endocrinologica. 38 (1): 73–87. doi:10.1530/acta.0.0380073. PMID 13759555.
- Bachmann FF (January 1971). "[Treatment of menopausal complants with polyoestriol-phosphate. Experiences with Gynäsan injections]" [Treatment of menopausal complaints with polyoestriol-phosphate. Experiences with Gynäsan injections]. Munchener Medizinische Wochenschrift (in German). 113 (5): 166–169. PMID 5107471.
- Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548, 551. ISBN 978-3-642-96158-8.
The polymer of estradiol or estriol and phosphoric acid has an excellent depot action when given intramuscularly (polyestriol phosphate or polyestradiol phosphate) (Table 16). Phosphoric acid combines with the estrogen molecule at C3 and C17 to form a macromolecule. The compound is stored in the liver and spleen where the estrogen is steadily released by splitting off of the phosphate portion due to the action of alkaline phosphatase. [...] Conjugated estrogens and polyestriol and estradiol phosphate can also be given intravenously in an aqueous solution. Intravenous administration of ovarian hormones offers no advantages, however, and therefore has no practical significance. [...] The following duarations of action have been obtained with a single administration (WlED, 1954; LAURITZEN, 1968): [...] 50 mg polyestradiol phosphate ~ 1 month; 50 mg polyestriol phosphate ~ 1 month; 80 mg polyestriol phosphate ~ 2 months.
- Campbell S (6 December 2012). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. Springer Science & Business Media. pp. 395–. ISBN 978-94-011-6165-7.
In the Federal Republic of Germany between 10 and 20% of all climacteric women are on estrogen treatment. We have the following oral estrogens for a treatment. (t) Conjugated estrogens, (2) estradiol valerate, (3) ethinyl-estradiol and its cyclopentyl-enol ether, (4) stilbestrol, (5) ethinyl-estradiol-methyltestosterone, (6) estriol and estriol succinate, most of them as coated tablets. Several long acting injectable preparations are available: several esters of combined estradiol-testosterone, one of estradiol-dehydroepiandrosterone enanthate and a prolonged polyestriol phosphate are also available. Lastly, depot injections of estradiol- and stilbestrol-esters are on the market.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1481–. ISBN 978-0-8155-1856-3.
- Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 114–. ISBN 978-94-011-4439-1.
- "Estriol succinate - Synthetic Biologics - AdisInsight".