Fluoroform
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a Hydrofluorocarbon as well as being apart of the haloforms, a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.[2]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trifluoromethane | |||
| Other names
Fluoroform, carbon trifluoride, methyl trifluoride, Fluoryl, Freon 23, Arcton 1, HFC 23, R-23, FE-13, UN 1984 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.794 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CHF3 | |||
| Molar mass | 70.014 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 2.946 kg/m3 (gas, 1 bar, 15 °C) | ||
| Melting point | −155.2 °C (−247.4 °F; 118.0 K) | ||
| Boiling point | −82.1 °C (−115.8 °F; 191.1 K) | ||
| 1 g/l | |||
| Solubility in organic solvents | Soluble | ||
| Vapor pressure | 4.38 MPa at 20 °C | ||
Henry's law constant (kH) |
0.013 mol·kg−1·bar−1 | ||
| Acidity (pKa) | 25–28 | ||
| Structure | |||
| Tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Nervous system depression | ||
| GHS labelling:[1] | |||
 | |||
| Warning | |||
| H280 | |||
| P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Related compounds |
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Synthesis
About 20 million kg per year are produced industrially as both a by-product of and precursor to the manufacture of Teflon.[2] It is produced by reaction of chloroform with HF:[3]
- CHCl3 + 3 HF → CHF3 + 3 HCl
It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid.[4]
Historical
Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894.[5] The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of mercury fluoride and calcium fluoride.[6] The exchange reaction works with iodoform and bromoform, and the exchange of the first two halogen atoms by fluorine is vigorous. By changing to a two step process, first forming a bromodifluoromethane in the reaction of antimony trifluoride with bromoform and finishing the reaction with mercury fluoride the first efficient synthesis method was found by Henne.[6]
Industrial applications
CHF3 is used in the semiconductor industry in plasma etching of silicon oxide and silicon nitride. Known as R-23 or HFC-23, it was also a useful refrigerant, sometimes as a replacement for chlorotrifluoromethane (CFC-13) and is a byproduct of its manufacture.
When used as a fire suppressant, the fluoroform carries the DuPont trade name, FE-13. CHF3 is recommended for this application because of its low toxicity, its low reactivity, and its high density. HFC-23 has been used in the past as a replacement for Halon 1301[CFC-13B1] in fire suppression systems as a total flooding gaseous fire suppression agent.
Organic chemistry
Fluoroform is weakly acidic with a pKa = 25–28 and quite inert. Attempted deprotonation results in defluorination to generate F− and difluorocarbene (CF2). Some organocopper and organocadmium compounds have been developed as trifluoromethylation reagents.[7]
Fluoroform is a precursor of the Ruppert-Prakash reagent CF3Si(CH3)3, which is a source of the nucleophilic CF−3 anion.[8][9]
Greenhouse gas
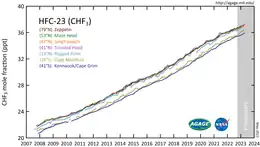
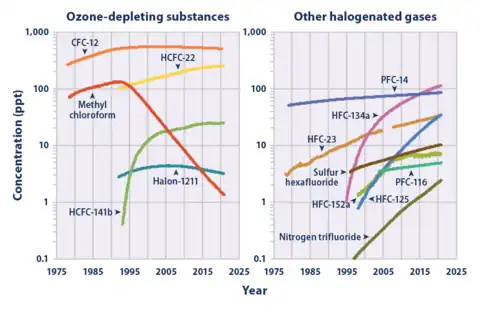
CHF3 is a potent greenhouse gas. A ton of HFC-23 in the atmosphere has the same effect as 11,700 tons of carbon dioxide. This equivalency, also called a 100-yr global warming potential, is slightly larger at 14,800 for HFC-23.[10] The atmospheric lifetime is 270 years.[10]
HFC-23 was the most abundant HFC in the global atmosphere until around 2001, when the global mean concentration of HFC-134a (1,1,1,2-tetrafluoroethane), the chemical now used extensively in automobile air conditioners, surpassed those of HFC-23. Global emissions of HFC-23 have in the past been dominated by the inadvertent production and release during the manufacture of the refrigerant HCFC-22 (chlorodifluoromethane).
Substantial decreases in HFC-23 emissions by developed countries were reported from the 1990s to the 2000s: from 6-8 Gg/yr in the 1990s to 2.8 Gg/yr in 2007.[11]
The UNFCCC Clean Development Mechanism provided funding and facilitated the destruction of HFC-23.
Developing countries have become the largest producers of HCFC-23 in recent years according to data compiled by the Ozone Secretariat of the World Meteorological Organization.[12][13][14] Emissions of all HFCs are included in the UNFCCCs Kyoto Protocol. To mitigate its impact, CHF3 can be destroyed with electric plasma arc technologies or by high temperature incineration.[15]
Additional physical properties
| Property | Value |
|---|---|
| Density (ρ) at -100 °C (liquid) | 1.52 g/cm3 |
| Density (ρ) at -82.1 °C (liquid) | 1.431 g/cm3 |
| Density (ρ) at -82.1 °C (gas) | 4.57 kg/m3 |
| Density (ρ) at 0 °C (gas) | 2.86 kg/m3 |
| Density (ρ) at 15 °C (gas) | 2.99 kg/m3 |
| Dipole moment | 1.649 D |
| Critical pressure (pc) | 4.816 MPa (48.16 bar) |
| Critical temperature (Tc) | 25.7 °C (299 K) |
| Critical density (ρc) | 7.52 mol/l |
| Compressibility factor (Z) | 0.9913 |
| Acentric factor (ω) | 0.26414 |
| Viscosity (η) at 25 °C | 14.4 μPa.s (0.0144 cP) |
| Molar specific heat at constant volume (CV) | 51.577 J.mol−1.K−1 |
| Latent heat of vaporization (lb) | 257.91 kJ.kg−1 |
References
- GHS: GESTIS 038260
- ShivaKumar Kyasa (2015). "Fluoroform (CHF3)". Synlett. 26 (13): 1911–1912. doi:10.1055/s-0034-1380924.
- G. Siegemund; W. Schwertfeger; A. Feiring; B. Smart; F. Behr; H. Vogel; B. McKusick (2005). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- Kirschner, E., Chemical and Engineering News 1994, 8.
- Meslans M. M. (1894). "Recherches sur quelques fluorures organiques de la série grasse". Annales de chimie et de physique. 7 (1): 346–423.
- Henne A. L. (1937). "Fluoroform". Journal of the American Chemical Society. 59 (7): 1200–1202. doi:10.1021/ja01286a012.
- Zanardi, Alessandro; Novikov, Maxim A.; Martin, Eddy; Benet-Buchholz, Jordi; Grushin, Vladimir V. (2011-12-28). "Direct Cupration of Fluoroform". Journal of the American Chemical Society. 133 (51): 20901–20913. doi:10.1021/ja2081026. ISSN 0002-7863. PMID 22136628.
- Rozen, S.; Hagooly, A. "Fluoroform" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rn00522
- Prakash, G. K. Surya; Jog, Parag V.; Batamack, Patrice T. D.; Olah, George A. (2012-12-07). "Taming of Fluoroform: Direct Nucleophilic Trifluoromethylation of Si, B, S, and C Centers". Science. 338 (6112): 1324–1327. Bibcode:2012Sci...338.1324P. doi:10.1126/science.1227859. ISSN 0036-8075. PMID 23224551. S2CID 206544170.
- Forster, P.; V. Ramaswamy; P. Artaxo; T. Berntsen; R. Betts; D.W. Fahey; J. Haywood; J. Lean; D.C. Lowe; G. Myhre; J. Nganga; R. Prinn; G. Raga; M. Schulz & R. Van Dorland (2007). "Changes in Atmospheric Constituents and in Radiative Forcing." (PDF). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.
- Montzka, S. A.; Kuijpers, L.; Battle, M. O.; Aydin, M.; Verhulst, K. R.; Saltzman, E. S.; Fahey, D. W. (2010). "Recent increases in global HFC-23 emissions". Geophysical Research Letters. 37 (2): n/a. Bibcode:2010GeoRL..37.2808M. doi:10.1029/2009GL041195. S2CID 13583576.
- "Data Access Centre". Archived from the original on 2011-07-21. Retrieved 2010-04-03.
- Profits on Carbon Credits Drive Output of a Harmful Gas August 8, 2012 New York Times
- Subsidies for a Global Warming Gas
- Han, Wenfeng; Li, Ying; Tang, Haodong; Liu, Huazhang (2012). "Treatment of the potent greenhouse gas, CHF3. An overview". Journal of Fluorine Chemistry. 140: 7–16. doi:10.1016/j.jfluchem.2012.04.012.
Literature
- McBee E. T. (1947). "Fluorine Chemistry". Industrial & Engineering Chemistry. 39 (3): 236–237. doi:10.1021/ie50447a002.
- Oram D. E.; Sturges W. T.; Penkett S. A.; McCulloch A.; Fraser P. J. (1998). "Growth of fluoroform (CHF3, HFC-23) in the background atmosphere". Geophysical Research Letters. 25 (1): 236–237. Bibcode:1998GeoRL..25...35O. doi:10.1029/97GL03483.
- McCulloch A. (2003). "Fluorocarbons in the global environment: a review of the important interactions with atmospheric chemistry and physics". Journal of Fluorine Chemistry. 123 (1): 21–29. doi:10.1016/S0022-1139(03)00105-2.