Stress granule
In cellular biology, stress granules are biomolecular condensates in the cytosol composed of proteins and RNAs that assemble into 0.1–2 μm membraneless organelles when the cell is under stress.[1][2][3] The mRNA molecules found in stress granules are stalled translation pre-initiation complexes associated with 40S ribosomal subunits, translation initiation factors, poly(A)+ mRNAs and RNA-binding proteins (RBPs). While they are membraneless organelles, stress granules have been proposed to be associated with the endoplasmatic reticulum.[4] There are also nuclear stress granules. This article is about the cytosolic variety.
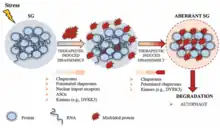
Proposed functions
The function of stress granules remains largely unknown. Stress granules have long been proposed to have a function to protect RNAs from harmful conditions, thus their appearance under stress.[5] The accumulation of RNAs into dense globules could keep them from reacting with harmful chemicals and safeguard the information coded in their RNA sequence.
Stress granules might also function as a decision point for untranslated mRNAs. Molecules can go down one of three paths: further storage, degradation, or re-initiation of translation.[6] Conversely, it has also been argued that stress granules are not important sites for mRNA storage nor do they serve as an intermediate location for mRNAs in transit between a state of storage and a state of degradation.[7]
Efforts to identify all RNAs within stress granules (the stress granule transcriptome) in an unbiased way by sequencing RNA from biochemically purified stress granule "cores" have shown that RNAs are not recruited to stress granules in a sequence-specific manner, but rather generically, with longer and/or less-optimally translated transcripts being enriched.[8] These data imply that the stress granule transcriptome is influenced by the valency of RNA (for proteins or other RNAs) and by the rates of RNA run-off from polysomes. The latter is further supported by recent single molecule imaging studies.[9] Furthermore, it was estimated that only about 15% of the total mRNA in the cell is localized to stress granules,[8] suggesting that stress granules only influence a minority of mRNAs in the cell and may not be as important for mRNA processing as previously thought.[8][10] That said, these studies represent only a snapshot in time, and it is likely that a larger fraction of mRNAs are at one point stored in stress granules due to those RNAs transiting in and out.
The stress proteins that are the main component of stress granules in plant cells are molecular chaperones that sequester, protect, and possibly repair proteins that unfold during heat and other types of stress.[11][12] Therefore, any association of mRNAs with stress granules may simply be a side effect of the association of partially unfolded RNA-binding proteins with stress granules,[13] similar to the association of mRNAs with proteasomes.[14]
Formation
Environmental stressors trigger cellular signaling, eventually leading to the formation of stress granules. In vitro, these stressors can include heat, cold, oxidative stress (sodium arsenite), endoplasmic reticulum stress (thapsigargin), proteasome inhibition (MG132), hyperosmotic stress, ultraviolet radiation, inhibition of eIF4A (pateamine A, hippuristanol, or RocA), nitric oxide accumulation after treatment with 3-morpholinosydnonimine (SIN-1),[15] perturbation of pre-mRNA splicing,[16] and other stressors, like puromycin, which result in disassembled polysomes.[17] Many of these stressors result in the activation of particular stress-associated kinases (HRI, PERK, PKR, and GCN2), translational inhibition and stress granule formation.[17] Stress granules will also form upon Gαq activation in a mechanism that involves the release of stress granule associated proteins from the cytosolic population of the Gαq effector phospholipase Cβ.[18]
Stress granule formation is often downstream of the stress-activated phosphorylation of eukaryotic translation initiation factor eIF2α; this does not hold true for all types of stressors that induce stress granules,[17] for instance, eIF4A inhibition. Further downstream, prion-like aggregation of the protein TIA-1 promotes the formation of stress granules. The term prion-like is used because aggregation of TIA-1 is concentration dependent, inhibited by chaperones, and because the aggregates are resistant to proteases.[19] It has also been proposed that microtubules play a role in the formation of stress granules, perhaps by transporting granule components. This hypothesis is based on the fact that disruption of microtubules with the chemical nocodazole blocks the appearance of the granules.[20] Furthermore, many signaling molecules have been shown to regulate the formation or dynamics of stress granules; these include the "master energy sensor" AMP-activated protein kinase (AMPK),[21] the O-GlcNAc transferase enzyme (OGT),[22] and the pro-apoptotic kinase ROCK1.[23]
Potential roles of RNA-RNA interactions
RNA phase transitions driven in part by intermolecular RNA-RNA interactions may play a role in stress granule formation. Similar to intrinsically disordered proteins, total RNA extracts are capable of undergoing phase separation in physiological conditions in vitro.[24] RNA-seq analyses demonstrate that these assemblies share a largely overlapping transcriptome with stress granules,[24][8] with RNA enrichment in both being predominately based on the length of the RNA. Further, stress granules contain many RNA helicases,[25] including the DEAD/H-box helicases Ded1p/DDX3, eIF4A1, and RHAU.[26] In yeast, catalytic ded1 mutant alleles give rise to constitutive stress granules[27] ATPase-deficient DDX3X (the mammalian homolog of Ded1) mutant alleles are found in pediatric medulloblastoma,[28] and these coincide with constitutive granular assemblies in patient cells.[29] These mutant DDX3 proteins promote stress granule assembly in HeLa cells.[29] In mammalian cells, RHAU mutants lead to reduced stress granule dynamics.[26] Thus, some hypothesize that RNA aggregation facilitated by intermolecular RNA-RNA interactions plays a role in stress granule formation, and that this role may be regulated by RNA helicases.[30] There is also evidence that RNA within stress granules is more compacted, compared to RNA in the cytoplasm, and that the RNA is preferentially post-translationally modified by N6-methyladenosine (m6A) on its 5' ends.[31][32] Recent work has shown that the highly abundant translation initiation factor and DEAD-box protein eIF4A limits stress granule formation. It does so through its ability to bind ATP and RNA, acting analogously to protein chaperones like Hsp70.[33]
Connection with processing bodies
Stress granules and P-bodies (processing bodies) share RNA and protein components, both appear under stress, and can physically associate with one another. As of 2018, of the ~660 proteins identified as localizing to stress granules, ~11% also have been identified as processing body-localized proteins (see below). The protein G3BP1 is necessary for the proper docking of processing bodies and stress granules to each other, which may be important for the preservation of polyadenylated mRNAs.[34]
Although some protein components are shared between stress granules and processing bodies, the majority of proteins in either structure are uniquely localized to either structure.[35] While both stress granules and P-bodies are associated with mRNAs, processing bodies have been long proposed to be sites of mRNA degradation because they contain enzymes like DCP1/2 and XRN1 that are known to degrade mRNAs.[36] However, others have demonstrated that mRNAs associated with processing bodies are largely translationally repressed but not degraded.[35] It has also been proposed that mRNAs selected for degradation are passed from stress granules to processing bodies,[36] though there is also data suggesting that processing bodies precede and promote stress granule formation.[37]
Protein composition of stress granules
The complete proteome of stress granules is still unknown, but efforts have been made to catalog all of the proteins that have been experimentally demonstrated to transit into stress granules.[38][39][40] Importantly, different stressors can result in stress granules with different protein components.[17] Many stress granule-associated proteins have been identified by transiently stressing cultured cells and utilizing microscopy to detect the localization of a protein of interest either by expressing that protein fused to a fluorescent protein (i.e. green fluorescent protein (GFP)) and/or by fixing cells and using antibodies to detect the protein of interest along with known protein markers of stress granules (immunocytochemistry).[41]
In 2016, stress granule "cores" were experimentally identified and then biochemically purified for the first time. Proteins in the cores were identified in an unbiased manner using mass spectrometry. This technical advance lead to the identification of hundreds of new stress granule-localized proteins.[42][25][43]
The proteome of stress granules has also been experimentally determined by using two slightly different proximity labeling approaches. One of these proximity labeling approaches is the ascorbate peroxidase (APEX) method, in which cells are engineered to express a known stress granule protein, such as G3BP1, fused to a modified ascorbate peroxidase enzyme called APEX.[38][44] Upon incubating the cells in biotin and treating the cells with hydrogen peroxide, the APEX enzyme will be briefly activated to biotinylate all proteins in close proximity to the protein of interest, in this case G3BP1 within stress granules. Proteins that are biotinylated can then be isolated via streptavidin and identified using mass spectrometry. The APEX technique was used to identify ~260 stress granule-associated proteins in several cell types, including neurons, and with various stressors. Of the 260 proteins identified in this study, ~143 had not previously been demonstrated to be stress granule-associated.[44]
Another proximity labeling method used to determine the proteome of stress granules is BioID.[45] BioID is similar to the APEX approach, in that a biotinylating protein (BirA* instead of APEX) was expressed in cells as a fusion protein with several known stress granule-associated proteins. Proteins in close proximity to BirA* will be biotinylated and are then identified by mass spectrometry. Youn et al. used this method to identify/predict 138 proteins as stress granule-associated and 42 as processing body-associated.[45]
A curated database of stress granule-associated proteins can be found here .[40]
The following is a list of proteins that have been demonstrated to localize to stress granules (compiled from [38][39][25][44][45][46]):
| Gene ID | Protein Name | Description | References | Also found in processing bodies? |
|---|---|---|---|---|
| ABCF1 | ABCF1 | ATP Binding Cassette Subfamily F Member 1 | [44] | |
| ABRACL | ABRACL | ABRA C-Terminal Like | [44] | |
| ACAP1 | ACAP1 | ArfGAP With Coiled-Coil, Ankyrin Repeat And PH Domains 1 | [44] | |
| ACBD5 | ACBD5 | Acyl-CoA Binding Domain Containing 5 | [44] | |
| ACTBL2 | ACTBL2 | Beta-actin-like protein 2 | [25] | yes[35] |
| ACTR1A | ACTR1A | Alpha-centractin | [25] | |
| ACTR1B | ACTR1B | Beta-centractin | [25] | |
| ADAR | ADAR1 | Adenosine Deaminase, RNA Specific | [47][25] | |
| ADD1 | Adducin 1 | Adducin 1 | [44] | |
| AGO1 | Argonaute 1/EIF2C1 | Argonaute 1, RISC Catalytic Component | [44][48] | yes[35] |
| AGO2 | Argonaute 2 | Argonaute 2, RISC Catalytic Component | [44][49][48][50][25][51][46] | yes[35] |
| AKAP8 | AKAP8 | A-Kinase Anchoring Protein 8 | [46] | |
| AKAP9 | AKAP350 | A-Kinase Anchoring Protein 9 | [52] | |
| AKAP13 | AKAP13/LBC | A-Kinase Anchoring Protein 13 | [44][46] | |
| ALDH18A1 | ALDH18A1 | Delta-1-pyrroline-5-carboxylate synthase | [25] | |
| ALG13 | ALG13 | ALG13, UDP-N-Acetylglucosaminyltransferase Subunit | [45] | |
| ALPK2 | ALPK2/HAK | Alpha Kinase 2 | [46] | |
| AMOTL2 | AMOTL2/LCCP | Angiomotin Like 2 | [46] | |
| ANKHD1 | ANKHD1 | Ankyrin Repeat and KH Domain Containing 1 | [45] | yes[45] |
| ANKRD17 | ANKRD17/MASK2/GTAR | Ankyrin Repeat Domain 17 | [44][45] | yes[45] |
| ANG | Angiogenin | Angiogenin | [53] | |
| ANP32E | ANP32E | Acidic leucine-rich nuclear phosphoprotein 32 family member E | [25] | |
| ANXA1 | ANXA1 | Annexin A1 | [25] | |
| ANXA11 | ANXA11 | Annexin 11 | [44] | |
| ANXA6 | ANXA6 | Annexin 6 | [25] | |
| ANXA7 | ANXA7 | Annexin 7 | [25][44] | |
| APEX1 | APEX1 | DNA-(apurinic or apyrimidinic site) lyase | [25] | |
| APOBEC3C | APOBEC3C | Apolipoprotein B mRNA Editing Enzyme Catalytic Subunit 3C | [44][46] | |
| APOBEC3G | APOBEC3G | Apolipoprotein B mRNA Editing Enzyme Catalytic Subunit 3G | [48] | |
| ARID2 | ARID2/BAF200 | AT-Rich Interaction Domain 2 | [46] | |
| ARPC1B | ARPC1B | Actin-related protein 2/3 complex subunit 1B | [25] | |
| AHSA1 | AHA1 | Activator Of HSP90 ATPase Activity 1 | [54] | |
| AQR | AQR/IBP160 | Aquarius Intron-Binding Spliceosomal Factor | [44] | |
| ARMC6 | ARMC6 | Armadillo Repeat Containing 6 | [44] | |
| ASCC1 | ASCC1 | Activating Signal Cointegrator 1 Complex Subunit 1 | [44][45] | |
| ASCC3 | ASCC3 | Activating Signal Cointegrator 1 Complex Subunit 3 | [45] | |
| ATAD2 | ATAD2 | ATPase family AAA domain-containing protein 2 | [25] | |
| ATAD3A | ATAD3A | ATPase family AAA domain-containing protein 3A | [25] | yes[35] |
| ATG3 | ATG3 | Autophagy Related 3 | [44] | |
| ATP5A1 | ATP5A1 | ATP synthase subunit alpha, mitochondrial | [25] | |
| ATP6V1G1 | ATP6V1G1/ATP6G | ATPase H+ Transporting V1 Subunit G1 | [44] | |
| ATXN2 | Ataxin 2 | Ataxin 2 | [25][44][45][46][55][56][57][58][59][60] | |
| ATXN2L | Ataxin-2 like | Ataxin 2 Like | [25][44][45][46][57][60] | |
| BAG3 | BAG3 | BAG family molecular chaperone regulator 3 | [25] | |
| BANF1 | BANF1 | Barrier-to-autointegration factor | [25] | |
| BAZ1B | BAZ1B | Bromodomain Adjacent To Zinc Finger Domain 1B | [46] | |
| BAZ2A | BAZ2A | Bromodomain Adjacent To Zinc Finger Domain 2A | [46] | |
| BCCIP | BCCIP | BRCA2 And CDKN1A Interacting Protein | [44] | |
| BCLAF1 | BCLAF1 | BCL2 Associated Transcription Factor 1 | [44] | |
| BICC1 | BICC1 | BicC Family RNA Binding Protein 1 | [45] | |
| BIRC2 | BIRC2/CIAP1 | Baculoviral IAP Repeat Containing 2 | [46] | |
| BLM | BLM | BLM RecQ Like Helicase | [46] | |
| BOD1L1 | BOD1L1/FAM44A | Biorientation Of Chromosomes In Cell Division 1 Like 1 | [46] | |
| BOLL | BOULE | Boule Homolog, RNA Binding Protein | [61] | |
| BRAT1 | BRAT1 | BRCA1-associated ATM activator 1 | [25] | |
| BRF1 | BRF1 | BRF1, RNA Polymerase III Transcription Initiation Factor Subunit | [36] | |
| BTG3 | BTG3 | BTG Anti-Proliferation Factor 3 | [45] | yes[45] |
| C9orf72 | C9orf72 | Uncharacterized protein C9orf72 | [62][63] | |
| C15orf52 | C15orf52 | Uncharacterized protein C15orf52 | [25] | |
| C20orf27 | C20orf72 | Chromosome 20 Open Reading Frame 27 | [44] | |
| C2CD3 | C2CD3 | C2 Calcium Dependent Domain Containing 3 | [44] | |
| CALML5 | CALML5 | Calmodulin-like protein 5 | [25] | |
| CALR | Calreticulin/CRT | Calreticulin | [64] | |
| CAMSAP1 | CAMSAP1 | Calmodulin Regulated Spectrin Associated Protein 1 | [46] | |
| CAP1 | CAP1 | Adenylyl cyclase-associated protein 1 | [25] | |
| CAPRIN1 | Caprin-1 | Cell Cycle Associated Protein 1 | [44][45][65][52][66][25][67][34][68][60][46] | |
| CAPZA2 | CAPZA2 | F-actin-capping protein subunit alpha-2 | [25] | |
| CAPZB | CAPZB | Capping Actin Protein Of Muscle Z-Line Subunit Beta | [46] | |
| CARHSP1 | CARHSP1 | Calcium-regulated heat stable protein 1 | [25] | |
| CASC3 | MLN51/BTZ | Cancer Susceptibility 3 | [44][45][46][69][70] | |
| CBFB | CBFB | Core-binding factor subunit beta | [25] | |
| CBS | CBS | Cystathionine Beta-Synthase | [46] | |
| CBX1 | CBX1 | Chromobox protein homolog 1 | [25][60] | |
| CBX3 | CBX3 | Chromobox protein homolog 3 | [46] | |
| CCAR1 | CARP-1 | Cell Division Cycle and Apoptosis Regulator 1 | [52][46] | |
| CCDC9 | CCDC9 | Coiled-Coil Domain Containing 9 | [46] | |
| CCDC9B | CCDC9B | Coiled-Coil Domain Containing 9B | [46] | |
| CCDC124 | CCDC124 | Coiled-Coil Domain Containing 124 | [44] | |
| CCDC85C | CCDC85C | Coiled-Coil Domain Containing 85C | [44] | |
| CCT3 | CCT3 | T-complex protein 1 subunit gamma | [25] | |
| CCT6A | CCT6A | T-complex protein 1 subunit zeta | [25] | |
| CDC20 | CDC20 | Cell Division Cycle 20 | [46] | |
| CDC37 | CDC37 | Cell Division Cycle 37 | [54] | |
| CDC5L | CDC5L | Cell division cycle 5-like protein | [25] | |
| CDC73 | CDC73 | Parafibromin | [25] | |
| CDK1 | CDK1 | Cyclin-dependent kinase 1 | [25] | |
| CDK2 | CDK2 | Cyclin Dependent Kinase 2 | [71] | |
| CDV3 | CDV3 | CDV3 Homolog | [44] | |
| CELF1 | CUGBP1 | CUGBP Elav-Like Family Member 1 | [25][44][45][46][72] | |
| CELF2 | CUGBP2/BRUNOL3 | CUGBP Elav-Like Family Member 2 | [44] | |
| CELF3 | CUGBP3/BRUNOL1 | CUGBP Elav-Like Family Member 3 | [44] | |
| CENPB | CENPB | Major centromere autoantigen B | [25] | |
| CENPF | CENPF | Centromere Protein F | [46] | |
| CEP78 | CEP78/CRDHL | Centrosomal Protein 78 | [44] | |
| CEP85 | CEP85/CCDC21 | Centrosomal Protein 78 | [45] | |
| CERKL | Ceramide-Kinase Like | Ceramide Kinase Like | [73] | |
| CFL1 | Cofilin-1 | Cofilin-1 | [25] | |
| CHCHD3 | CHCHD3 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 3, mitochondrial | [25] | |
| CHORDC1 | CHORDC1/CHP1 | Cysteine and histidine-rich domain-containing protein 1 | [25] | |
| CIRBP | CIRP | Cold Inducible RNA Binding Protein | [44][46][74] | |
| CIT | CIT | Citron Rho-interacting kinase | [25] | |
| CLIC4 | CLIC4 | Chloride intracellular channel protein 4 | [25] | |
| CLNS1A | CLNS1A | Chloride Nucleotide-Sensitive Channel 1A | [44] | |
| CLPP | CLPP | Caseinolytic Mitochondrial Matrix Peptidase Proteolytic Subunit | [44] | |
| CNBP | ZNF9 | CCHC-Type Zinc Finger Nucleic Acid Binding Protein | [46][75] | |
| CNN3 | CNN3 | Calponin-3 | [25] | |
| CNOT1 | CNOT1/CCR4 | CCR4-Not Transcription Complex Subunit 1 | [25][45] | yes[45][76] |
| CNOT10 | CNOT10 | CCR4-Not Transcription Complex Subunit 10 | [45] | yes[45] |
| CNOT11 | CNOT11 | CCR4-Not Transcription Complex Subunit 11 | [45] | yes[45] |
| CNOT2 | CNOT2 | CCR4-Not Transcription Complex Subunit 2 | [45] | yes[45] |
| CNOT3 | CNOT3 | CCR4-Not Transcription Complex Subunit 3 | [45] | yes[45] |
| CNOT4 | CNOT4 | CCR4-Not Transcription Complex Subunit 4 | [45] | yes[45] |
| CNOT6 | CNOT6 | CCR4-Not Transcription Complex Subunit 6 | [45] | yes[45] |
| CNOT6L | CNOT6L | CCR4-Not Transcription Complex Subunit 6L | [45] | yes[45] |
| CNOT7 | CNOT7 | CCR4-Not Transcription Complex Subunit 7 | [45] | yes[45] |
| CNOT8 | CNOT8 | CCR4-Not Transcription Complex Subunit 8 | [45] | yes[45] |
| CNOT9 | CNOT9 | CCR4-Not Transcription Complex Subunit 9 | [45] | |
| CORO1B | CORO1B | Coronin-1B | [25] | |
| CPB2 | Carboxypeptidase B2 | Carboxypeptidase B2 | [77] | |
| CPEB1 | CPEB | Cytoplasmic Polyadenylation Element Binding Protein 1 | [78] | |
| CPEB4 | CPEB4 | Cytoplasmic Polyadenylation Element Binding Protein 4 | [44][45][46] | yes[45] |
| CPSF3 | CPSF3 | Cleavage and polyadenylation specificity factor subunit 3 | [25] | |
| CPSF6 | CPSF6 | Cleavage and polyadenylation specificity factor subunit 6 | [25] | |
| CPSF7 | CPSF7 | Cleavage and polyadenylation specificity factor subunit 7 | [25] | |
| CPVL | CPVL | Carboxypeptidase, Vitellogenic Like | [45] | yes[45] |
| CRKL | CRKL | CRK Like Proto-Oncogene, Adaptor Protein | [44] | |
| CROCC | CROCC | Ciliary Rootlet Coiled-Coil, Rootletin | [44] | |
| CRYAB | CRYAB | Alpha-crystallin B chain | [25] | |
| CRYBG1 | CRYBG1 | Crystallin Beta-Gamma Domain Containing 1 | [46] | |
| CSDE1 | CSDE1 | Cold shock domain-containing protein E1 | [25][44][45][46][60] | |
| CSE1L | CSE1L/XPO2/Exportin-2 | Exportin-2 | [25] | |
| CSNK2A1 | Casein Kinase 2 alpha | Casein Kinase 2 Alpha 1 | [79] | |
| CSTB | Cystatin B | Cystatin B | [44] | |
| CSTF1 | CSTF1 | Cleavage stimulation factor subunit 1 | [25] | |
| CTNNA2 | CTNNA2 | Catenin alpha-2 | [25] | |
| CTNND1 | CTNND1 | Catenin delta-1 | [25] | |
| CTTNBP2NL | CTTNBP2NL | CTTNBP2 N-terminal-like protein | [25] | |
| CWC22 | CWC22 | Pre-mRNA-splicing factor CWC22 homolog | [25] | |
| DAZAP1 | DAZAP1 | DAZ-associated protein 1 | [25][44][45][46] | |
| DAZAP2 | PRTB | DAZ Associated Protein 2 | [80] | |
| DAZL | DAZL1 | Deleted In Azoospermia Like | [81] | |
| DCD | DCD | Dermcidin | [25] | |
| DCP1A | DCP1a | Decapping mRNA 1a | [25][44][78] | yes[35] |
| DCP1B | DCP1b | Decapping mRNA 1b | [44][46] | yes[35] |
| DCP2 | DCP2 | Decapping mRNA 2 | [45] | |
| DCTN1 | DCTN1 | Dynactin subunit 1 | [25] | |
| DDX1 | DEAD box protein 1 | DEAD-Box Helicase 1 | [25][44][45][46][82] | |
| DDX11 | DEAD box protein 11 | DEAD-Box Helicase 11 | [46] | |
| DDX19A | DDX19A | ATP-dependent RNA helicase DDX19A | [25][60] | |
| DDX21 | DDX21 | Nucleolar RNA helicase 2 | [25] | yes[35] |
| DDX3 | DEAD box protein 3 | DEAD-Box Helicase 3 | [25][83][84] | |
| DDX3X | DDX3X | DEAD-Box Helicase 3, X-Linked | [44][45][46][85][86][60] | |
| DDX3Y | DDX3Y | DEAD-Box Helicase 3, Y-Linked | [44] | |
| DDX31 | DDX31 | DEAD-Box Helicase 31 | [46] | |
| DDX47 | DDX47 | Probable ATP-dependent RNA helicase DDX47 | [25] | |
| DDX50 | DDX50 | ATP-dependent RNA helicase DDX50 | [25] | yes[35] |
| DDX58 | RIG-I | DExD/H-Box Helicase 58 | [87] | |
| DDX6 | DEAD box protein 6 | DEAD-Box Helicase 6 | [25][44][45][56][88][78][48][89][46] | yes[35][45] |
| DERA | DERA | Deoxyribose-Phosphate Aldolase | [90] | |
| DGCR8 | DGCR8 | DGCR8 Microprocessor Complex Subunit | [46] | |
| DHX30 | DHX30 | Putative ATP-dependent RNA helicase DHX30 | [25][44] | yes[35] |
| DHX33 | DHX33 | DEAH-Box Helicase 33 | [44] | |
| DHX36 | RHAU | DEAH-Box Helicase 36 | [44][45][26][46] | |
| DHX57 | DHX57 | DExH-Box Helicase 57 | [45][46] | |
| DHX58 | LGP2 | DExH-Box Helicase 58 | [87] | |
| DIDO1 | DIDO1 | Death Inducer-Obliterator 1 | [46] | |
| DIS3L2 | DIS3L2/FAM3A | DIS3 Like 3'-5' Exoribonuclease 2 | [44] | |
| DISC1 | Disrupted in Schizophrenia 1 | Disrupted In Schizophrenia 1 | [91] | |
| DKC1 | DKC1 | dyskerin; H/ACA ribonucleoprotein complex subunit 4 | [25][92] | |
| DNAI1 | Axonemal Dynein Intermediate Chain 1 | Dynein Axonemal Intermediate Chain 1 | [93] | |
| DNAJA1 | DNAJA1 | DnaJ homolog subfamily A member 1 | [25] | |
| DNAJC8 | DNAJC8 | DnaJ homolog subfamily C member 8 | [25] | |
| DOCK4 | DOCK4 | Dedicator Of Cytokinesis 4 | [46] | |
| DPYSL2 | DPYSL2 | Dihydropyrimidinase-related protein 2 | [25] | |
| DPYSL3 | DPYSL3 | Dihydropyrimidinase-related protein 3 | [25] | |
| DROSHA | DROSHA | Drosha Ribonuclease III | [44] | |
| DSP | DSP | Desmoplakin | [25][44] | |
| DST | DST | Dystonin | [25] | |
| DSTN | DSTN | Destrin | [25] | |
| DTL | DTL | Denticleless E3 Ubiquitin Protein Ligase Homolog | [46] | |
| DTX3L | DTX3L | E3 ubiquitin-protein ligase DTX3L | [25] | |
| DUSP12 | DUSP12/YVH1 | Dual Specificity Phosphatase 12 | [94] | |
| DYNC1H1 | Cytoplasmic Dynein Heavy Chain 1 | Dynein Cytoplasmic 1 Heavy Chain 1 | [93] | |
| DYNLL1 | Cytoplasmic Dynein Light Polypeptide | Dynein Light Chain LC8-Type 1 | [44][95] | |
| DYNLL2 | DYNLL2 | Dynein light chain 2, cytoplasmic | [25] | |
| DYRK3 | DYRK3 | Dual Specificity Tyrosine Phosphorylation Regulated Kinase 3 | [96] | |
| DZIP1 | DZIP1 | DAZ Interacting Zinc Finger Protein 1 | [97] | |
| DZIP3 | DZIP3 | DAZ Interacting Zinc Finger Protein 3 | [45] | |
| EDC3 | EDC3 | Enhancer of mRNA Decapping 3 | [44][45][46] | yes[45] |
| EDC4 | EDC4 | Enhancer of mRNA-Decapping protein 4 | [25][44][46] | yes[35] |
| EIF1 | EIF1 | Eukaryotic Translation Initiation Factor 1 | [44] | |
| EIF2A | EIF2A | Eukaryotic Translation Initiation Factor 2A | [36][25][52][98] | |
| EIF2AK2 | Protein Kinase R/PKR | Eukaryotic Translation Initiation Factor 2 Alpha Kinase 2 | [68][87][99] | |
| EIF2B1-5 | EIF2B | Eukaryotic Translation Initiation Factor 2B | [98] | |
| EIF2S1 | EIF2A subunit 1 | Eukaryotic Translation Initiation Factor 2 Subunit Alpha | [25] | |
| EIF2S2 | EIF2A subunit 2 | Eukaryotic Translation Initiation Factor 2 Subunit Beta | [25] | |
| EIF3A | EIF3A | Eukaryotic Translation Initiation Factor 3 Subunit A | [25][44][49][34][100][46] | |
| EIF3B | EIF3B | Eukaryotic Translation Initiation Factor 3 Subunit B | [36][25][80][101][102] | |
| EIF3C | EIF3C | Eukaryotic Translation Initiation Factor 3 Subunit C | [44] | |
| EIF3D | EIF3D | Eukaryotic translation initiation factor 3 subunit D | [25][44][60] | |
| EIF3E | EIF3E | Eukaryotic translation initiation factor 3 subunit E | [25][44][60] | |
| EIF3F | EIF3F | Eukaryotic translation initiation factor 3 subunit F | [25] | |
| EIF3G | EIF3G | Eukaryotic translation initiation factor 3 subunit G | [25][44][60][46] | |
| EIF3H | EIF3H | Eukaryotic translation initiation factor 3 subunit H | [25][44][46] | |
| EIF3I | EIF3I | Eukaryotic translation initiation factor 3 subunit I | [25][46] | |
| EIF3J | EIF3J | Eukaryotic translation initiation factor 3 subunit J | [25][44] | |
| EIF3K | EIF3K | Eukaryotic translation initiation factor 3 subunit K | [25] | |
| EIF3L | EIF3L | Eukaryotic translation initiation factor 3 subunit L | [25][44][60] | |
| EIF3M | EIF3M | Eukaryotic translation initiation factor 3 subunit M | [25] | |
| EIF4A1 | EIF4A1 | Eukaryotic Translation Initiation Factor 4A1 | [25][44][103][46] | |
| EIF4A2 | EIF4A2 | Eukaryotic Translation Initiation Factor 4A2 | [44][104][46] | |
| EIF4A3 | EIF4A3 | Eukaryotic Translation Initiation Factor 4A3 | [44] | |
| EIF4B | EIF4B | Eukaryotic translation Initiation factor 4B | [25][44][46] | |
| EIF4E | EIF4E | Eukaryotic Translation Initiation Factor 4E | [100][98][4][105][70][106][107][36] | yes[36] |
| EIF4E2 | EIF4E2 | Eukaryotic Translation Initiation Factor 4E Family Member 2 | [45][107] | yes[45] |
| EIF4E3 | EIF4E3 | Eukaryotic Translation Initiation Factor 4E Family Member 3 | [107] | |
| EIF4ENIF1 | EIF4ENIF1 | Eukaryotic Translation Initiation Factor 4E Nuclear Import Factor 1 | [44][45] | yes[45] |
| EIF4G1 | EIF4G1 | Eukaryotic Translation Initiation Factor 4G1 | [25][44][100][98][4][105][108][109][80][110][34][46] | |
| EIF4G2 | EIF4G2 | Eukaryotic Translation Initiation Factor 4G2 | [25][45] | |
| EIF4G3 | EIF4G3 | Eukaryotic Translation Initiation Factor 4G3 | [44] | |
| EIF4H | EIF4H | Eukaryotic translation Initiation factor 4H | [25][44][46] | |
| EIF5A | EIF5A | Eukaryotic Translation Initiation Factor 5A | [101] | |
| ELAVL1 | HuR | ELAV Like RNA Binding Protein 1 | [25][34][44][111][100][112][105][106][80][95][113][114][46] | yes[35] |
| ELAVL2 | ELAVL2 | ELAV-like protein 2 | [25][44] | yes[35] |
| ELAVL3 | ELAVL3/HuC | ELAV Like RNA Binding Protein 3 | [44] | |
| ELAVL4 | HuD | ELAV Like RNA Binding Protein 4 | [44][115] | |
| ENC1 | ENC1 | Ectodermal-Neural Cortex 1 | [46] | |
| ENDOV | EndoV | Endonuclease V | [116] | |
| ENTPD1 | ENTPD1 | Ectonucleoside Triphosphate Diphosphohydrolase 1 | [44] | |
| EP400 | EP400 | E1A Binding Protein P400 | [46] | |
| EPPK1 | EPPK1 | Epiplakin | [25] | |
| ETF1 | ETF1 | Eukaryotic peptide chain release factor subunit 1 | [25] | |
| EWSR1 | EWSR1 | EWS RNA Binding Protein 1 | [117][118][46] | |
| FABP5 | FABP5 | Fatty Acid Binding Protein 5 | [44] | |
| FAM120A | FAM120A/OSSA | Constitutive coactivator of PPAR-gamma-like protein 1 | [25][44][45] | yes[35] |
| FAM120C | FAM120C | Family With Sequence Similarity 120C | [44][45] | |
| FAM168A | FAM168A | Family With Sequence Similarity 168 Member A | [46] | |
| FAM168B | FAM168B/MANI | Family With Sequence Similarity 168 Member B | [44] | |
| FAM83H | FAM83H | Family With Sequence Similarity 83 Member H | [46] | |
| FAM98A | FAM98A | Family With Sequence Similarity 98 Member A | [25][44][119][46] | |
| FAM98C | FAM98C | Family With Sequence Similarity 98 Member C | [46] | |
| FASTK | FAST | Fas Activated Serine/Threonine Kinase | [36] | yes[36] |
| FBL | FBL | rRNA 2-O-methyltransferase fibrillarin | [25] | |
| FBRSL1 | Fibrosin Like 1 | Fibrosin Like 1 | [45] | |
| FHL1 | FHL1 | Four and a half LIM domains protein 1 | [25] | |
| FKBP1A | FKBP1A | FKBP Prolyl Isomerase 1A | [46] | |
| FLNB | FLNB | Filamin-B | [25] | |
| FMR1 | FMRP | Fragile X Mental Retardation 1 | [23][25][44][45][69][70][105][120][121][94][60][46] | |
| FNDC3B | FNDC3B | Fibronectin type III domain-containing protein 3B | [25][45][46] | |
| FSCN1 | FSCN1 | Fascin | [25] | |
| FTSJ3 | FTSJ3 | pre-rRNA processing protein FTSJ3 | [25] | |
| FUBP1 | FUBP1 | Far Upstream Element Binding Protein 1 | [44][46] | |
| FUBP3 | FUBP3 | Far upstream element-binding protein 3 | [25][44][45][46] | |
| FUS | FUS | FUS RNA Binding Protein | [25][44][49][117][118][122][123][124][125][126][127][128][46] | |
| FXR1 | FXR1 | FMR1 Autosomal Homolog 1 | [25][44][45][120][105][106][129][46] | |
| FXR2 | FXR2 | FMR1 Autosomal Homolog 2 | [25][44][45][120][105][46] | |
| G3BP1 | G3BP1 | G3BP Stress Granule Assembly Factor 1 | [25][44][45][67][99][68][130][131][36][106][132][129][133][60][46] | |
| G3BP2 | G3BP2 | G3BP Stress Granule Assembly Factor 2 | [25][44][45][134][135][60][46] | |
| GABARAPL2 | GABARAPL2/GEF2/ATG8 | GABA Type A Receptor Associated Protein Like 2 | [44] | |
| GAK | GAK | Cyclin G Associated Kinase | [46] | |
| GAR1 | GAR1 | H/ACA Ribonucleoprotein Complex Subunit 1 | [92] | |
| GCA | Grancalcin | Grancalcin | [44] | |
| GEMIN5 | Gemin-5 | Gem Nuclear Organelle Associated Protein 5 | [108] | |
| GFPT1 | GFPT1 | Glutamine—fructose-6-phosphate aminotransferase [isomerizing] 1 | [25] | |
| GIGYF1 | GIGYF1/PERQ1 | GRB10 Interacting GYF Protein 1 | [44] | |
| GIGYF2 | GIGYF2/TNRC15/PARK11/PERQ2 | GRB10 Interacting GYF Protein 2 | [44][45] | yes[45] |
| GLE1 | GLE1 | GLE1, RNA Export Mediator | [45][136][137] | |
| GLO1 | Glyoxalase | Glyoxalase | [44] | |
| GLRX3 | GLRX3/Glutaredoxin 3/TNLX2 | Glutaredoxin 3 | [44] | |
| GLUD1 | GLUD1 | Glutamate Dehydrogenase 1 | [46] | |
| GNB2 | GNB2 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-2 | [25] | |
| GOLGA2 | Golgin A2 | Golgin A2 | [44] | |
| GPAT3 | GPAT3 | Glycerol-3-Phosphate Acyltransferase 3 | [46] | |
| GRB2 | GRB2/ASH | Growth Factor Receptor Bound Protein 2 | [44] | |
| GRB7 | GRB7 | Growth Factor Receptor Bound Protein 7 | [138][139] | |
| GRSF1 | GRSF1 | G-Rich RNA Sequence Binding Factor 1 | [44][45] | |
| GSPT1 | eRF3 | G1 To S Phase Transition 1 | [44][140] | |
| GTF2I | GTF2I | General Transcription Factor IIi | [46] | |
| GTF3C1 | GTF3C1 | General Transcription Factor IIIC Subunit 1 | [46] | |
| GTF3C4 | GTF3C4 | General Transcription Factor IIIC Subunit 4 | [46] | |
| H1F0 | H1F0 | Histone H1.0 | [25] | |
| H1FX | H1FX | Histone H1x | [25] | |
| H2AFV | H2AFV | Histone H2A.V | [25] | |
| HABP4 | Ki-1/57 | Hyaluronan Binding Protein 4 | [141] | |
| HDAC6 | HDAC6 | Histone Deacetylase 6 | [86][132][60] | |
| HDLBP | HDL-Binding Protein/VGL/Vigilin | High Density Lipoprotein Binding Protein | [44] | |
| HELZ | HELZ | Probable helicase with zinc finger domain | [25][44][45] | yes[45] |
| HELZ2 | HELZ2 | Helicase with zinc finger domain 2 | [25] | |
| HMGA1 | HMGA1 | High mobility group protein HMG-I/HMG-Y | [25] | |
| HMGB3 | HMGB3 | High mobility group protein B3 | [25] | |
| HMGN1 | HMGN1 | Non-histone chromosomal protein HMG-14 | [25] | |
| HNRNPA1 | HnRNPA1 | Heterogeneous Nuclear Ribonucleoprotein A1 | [25][44][49][142][143][144][145] | |
| HNRNPA2B1 | HnRNPA2/B1 | Heterogeneous Nuclear Ribonucleoprotein A2/B1 | [25][44][146][60] | |
| HNRNPA3 | HNRNPA3 | Heterogeneous nuclear ribonucleoprotein A3 | [25][44] | |
| HNRNPAB | HNRNPAB | Heterogeneous nuclear ribonucleoprotein A/B | [25][44][45] | |
| HNRNPD | HNRNPD | Heterogeneous nuclear ribonucleoprotein D | [44] | |
| HNRNPDL | HNRNPDL | Heterogeneous nuclear ribonucleoprotein D-like | [44] | |
| HNRNPF | HNRNPF | Heterogeneous nuclear ribonucleoprotein F | [44] | |
| HNRNPH1 | HNRNPH1 | Heterogeneous nuclear ribonucleoprotein H1 | [44] | |
| HNRNPH2 | HNRNPH2 | Heterogeneous nuclear ribonucleoprotein H2 | [25] | |
| HNRNPH3 | HNRNPH3 | Heterogeneous nuclear ribonucleoprotein H3 | [44] | |
| HNRNPK | HNRNPK | Heterogeneous Nuclear Ribonucleoprotein K | [25][114][147] | |
| HNRNPUL1 | HNRNPUL1 | Heterogeneous nuclear ribonucleoprotein U-like protein 2 | [25] | |
| HSBP1 | HSBP1 | Heat Shock Factor Binding Protein 1 | [44] | |
| HSP90AA1 | HSP90 | Heat shock protein HSP 90-alpha | [25] | |
| HSPA4 | HSP70 RY | Heat shock 70 kDa protein 4 | [25] | |
| HSPA9 | HSP70 9B | Stress-70 protein, mitochondrial | [25] | |
| HSPB1 | HSP27 | Heat Shock Protein Family B (Small) Member 1 | [25][148] | yes[35] |
| HSPB8 | HSPB8 | Heat Shock Protein Family B (Small) Member 8 | [149] | |
| HSPBP1 | HSPBP1 | HSPA (Hsp70) Binding Protein 1 | [150] | |
| HSPD1 | HSPD1 | 60 kDa heat shock protein, mitochondrial | [25][44] | |
| HTT | Huntingtin | Huntingtin | [66] | |
| IBTK | IBTK | Inhibitor Of Bruton Tyrosine Kinase | [45] | |
| IFIH1 | MDA5 | Interferon Induced With Helicase C Domain 1 | [87] | |
| IGF2BP1 | IGF2BP1 | Insulin-like Growth Factor 2 mRNA-binding protein 1 | [25][44][45] | yes[35] |
| IGF2BP2 | IGF2BP2 | Insulin-like Growth Factor 2 mRNA-binding protein 2 | [25][44][45] | yes[35] |
| IGF2BP3 | IGF2BP3 | Insulin-like Growth Factor 2 mRNA Binding Protein 3 | [25][44][45][134] | yes[35] |
| IK | IK | Protein Red | [25] | |
| ILF3 | NF90 | Interleukin Enhancer Binding Factor 3 | [151] | yes[35] |
| IPO7 | IPO7 | Importin-7 | [25] | |
| IPPK | IP5K | Inositol-Pentakisphosphate 2-Kinase | [152] | |
| ITGB1 | ITGB1 | Integrin beta-1 | [25] | |
| JMJD6 | JMJD6 | Arginine Demethylase and Lysine Hydroxylase | [133] | |
| KANK2 | KANK2 | KN motif and ankyrin repeat domain-containing protein 2 | [25] | |
| KEAP1 | KEAP1/KLHL19 | Kelch Like ECH Associated Protein 1 | [44] | |
| KHDRBS1 | Sam68 | KH RNA Binding Domain Containing, Signal Transduction Associated 1 | [25][153][154][155] | |
| KHDRBS3 | KHDRBS3 | KH domain-containing, RNA-binding, signal transduction-associated protein 3 | [25] | |
| KHSRP | KSRP/FBP2 | KH-Type Splicing Regulatory Protein | [25][44][156] | |
| KIAA0232 | KIAA0232 | KIAA0232 | [45] | yes[45] |
| KIAA1524 | CIP2A | Protein CIP2A | [25] | |
| KIF1B | KIF1B | Kinesin Family Member 1B | [45] | |
| KIF13B | KIF13B/GAKIN | Kinesin Family Member 13B | [44] | |
| KIF23 | KIF23 | Kinesin-like protein KIF23 | [25] | yes[35] |
| KIF2A | Kinesin Heavy Chain Member 2 | Kinesin Family Member 2A | [93] | |
| KLC1 | Kinesin Light Chain 1 | Kinesin Light Chain 1 | [93] | |
| KPNA1 | Importin-ɑ5 | Karyopherin Subunit Alpha 1 | [25][44][157] | |
| KPNA2 | Importin-ɑ1 | Karyopherin Subunit Alpha 2 | [25][157][158][137] | |
| KPNA3 | Importin-ɑ4 | Karyopherin Subunit Alpha 3 | [44][157] | |
| KPNA6 | Importin-ɑ7 | Importin subunit alpha | [25] | |
| KPNB1 | Importin-β1 | Karyopherin Subunit Beta 1 | [25][157][137][60] | |
| L1RE1 | LINE1 ORF1p | LINE1 ORF1 protein | [25][49] | |
| LANCL1 | LanC Like 1 | LanC Like 1 | [44] | |
| LARP1 | LARP1 | La-related protein 1 | [25] | |
| LARP1B | LARP1B | La-related protein 1b | [45] | |
| LARP4 | La-Related protein 4 | La Ribonucleoprotein Domain Family Member 4 | [25][44][45][159] | |
| LARP4B | LARP4B | La Ribonucleoprotein Domain Family Member 4B | [44][45] | |
| LASP1 | LIM And SH3 Protein 1/MLN50 | LIM And SH3 Protein 1 | [44] | |
| LBR | LBR | Lamin-B receptor | [25] | |
| LEMD3 | LEMD3 | Inner nuclear membrane protein Man1 | [25] | |
| LIG3 | DNA Ligase 3 | DNA Ligase 3 | [44] | |
| LIN28A | LIN28A | Lin-28 Homolog A | [44][160] | |
| LIN28B | LIN28B | Lin-28 Homolog B | [44][160] | |
| LMNA | LMNA | Prelamin-A/C | [25] | |
| LPP | LPP | Lipoma-preferred partner | [25] | |
| LSM1 | LSM1 | LSM1 Homolog, mRNA Degradation Associated | [44] | yes[161] |
| LSM12 | LSM12 | LSM12 Homolog | [44][45] | |
| LSM14A | RAP55 | LSM14A, mRNA Processing Body Assembly Factor | [25][44][45][162][163] | yes[35][45] |
| LSM14B | LSM14B | Protein LSM14 homolog B | [25][44][45] | yes[35] |
| LSM3 | LSM3 | U6 snRNA-associated Sm-like protein LSm3 | [25] | yes[161] |
| LUC7L | LUC7L | Putative RNA-binding protein Luc7-like 1 | [25] | |
| LUZP1 | LUZP1 | Leucine zipper protein 1 | [25][45] | |
| MACF1 | MACF1 | Microtubule-actin cross-linking factor 1, isoforms 1/2/3/5 | [25][60] | |
| MAEL | MAEL | Maelstrom Spermatogenic Transposon Silencer | [164] | |
| MAGEA4 | MAGEA4 | Melanoma-associated antigen 4 | [25] | |
| MAGED1 | MAGED1 | Melanoma-associated antigen D1 | [25][44][45] | |
| MAGED2 | MAGED2 | Melanoma-associated antigen D2 | [25] | |
| MAGOHB | MAGOHB | Protein mago nashi homolog 2 | [25] | |
| MAP1LC3A | LC3-I | Microtubule Associated Protein 1 Light Chain 3 Alpha | [165][166] | |
| MAP4 | MAP4 | Microtubule-associated protein 4 | [25] | |
| MAPK1IP1L | MAPK1IP1L | Mitogen-Activated Protein Kinase 1 Interacting Protein 1 Like | [44] | |
| MAP4K4 | MAP4K4 | Mitogen-activated protein kinase kinase kinase kinase 4 | [25] | |
| MAPK8 | JNK1 | Mitogen-Activated Protein Kinase 8 | [167] | |
| MAPRE1 | MAPRE1 | Microtubule-associated protein RP/EB family member 1 | [25] | |
| MAPRE2 | MAPRE2 | Microtubule Associated Protein RP/EB Family Member 2 | [44] | |
| MARF1 | MARF1 | Meiosis Regulator And mRNA Stability Factor 1 | [45] | yes[45] |
| MARS | MARS | Methionine—tRNA ligase, cytoplasmic | [25] | |
| MBNL1 | MBNL1 | Muscleblind Like Splicing Regulator 1 | [82] | |
| MBNL2 | MBNL2 | Muscleblind Like Splicing Regulator 2 | [45] | |
| MCM4 | MCM4 | DNA replication licensing factor MCM4 | [25] | |
| MCM5 | MCM5 | DNA replication licensing factor MCM5 | [25] | |
| MCM7 | MCM7 | DNA replication licensing factor MCM7 | [25] | yes[35] |
| METAP1 | METAP1 | Methionine aminopeptidase | [25] | |
| METAP2 | METAP2 | Methionyl Aminopeptidase 2 | [44] | |
| MCRIP1 | FAM195B/GRAN2 | Granulin-2 | [44][45][89] | |
| MCRIP2 | FAM195A/GRAN1 | Granulin-1 | [45][89] | |
| MEX3A | MEX3A | RNA-binding protein MEX3A | [25] | yes[35] |
| MEX3B | MEX3B | Mex-3 RNA Binding Family Member B | [44][168] | |
| MEX3C | MEX3C | Mex-3 RNA Binding Family Member C | [44][169] | |
| MEX3D | MEX3D | Mex-3 RNA Binding Family Member D | [45] | |
| MFAP1 | MFAP1 | Microfibrillar-associated protein 1 | [25] | |
| MKI67 | MKI67 | Antigen KI-67 | [25] | |
| MKRN2 | MKRN2 | Makorin Ring Finger Protein 2 | [44][45] | |
| MOV10 | MOV-10 | Mov10 RISC Complex RNA Helicase | [25][45][48] | yes[35][45] |
| MSH6 | MSH6 | DNA mismatch repair protein Msh6 | [25] | |
| MSI1 | Musashi-1 | Musashi RNA Binding Protein 1 | [44][163][170] | yes[35] |
| MSI2 | MSI2 | RNA-binding protein Musashi homolog 2 | [25][44] | |
| MTHFD1 | MTHFD1 | C-1-tetrahydrofolate synthase, cytoplasmic | [25] | |
| MTHFSD | MTHFSD | Methenyltetrahydrofolate Synthetase Domain Containing | [171] | |
| MTOR | MTOR | Mechanistic Target Of Rapamycin | [96][172] | |
| MYO6 | MYO6 | Unconventional myosin-VI | [25] | |
| NCOA3 | SRC-3 | Nuclear Receptor Coactivator 3 | [173] | |
| NDEL1 | NUDEL/MITAP1/EOPA | NudE Neurodevelopment Protein 1 Like 1 | [44] | |
| NELFE | NELF-E/RD | Negative Elongation Factor Complex Member E | [44] | |
| NEXN | NEXN | Nexilin | [25] | |
| NXF1 | NXF1/MEX67/TAP | Nuclear RNA Export Factor 1 | [45][60] | |
| NKRF | NRF | NFK-B Repressing Factor | [44] | |
| NOLC1 | Nucleolar And Coiled-Body Phosphoprotein 1/NOPP140 | Nucleolar And Coiled-Body Phosphoprotein 1 | [44] | |
| NONO | NonO | Non-POU Domain Containing Octamer Binding | [25][174] | |
| NOP58 | NOP58 | Nucleolar protein 58 | [25] | yes[35] |
| NOSIP | NOSIP | Nitric oxide synthase-interacting protein | [25] | |
| NOVA2 | NOVA2 | NOVA Alternative Splicing Regulator 2 | [44] | |
| NRG2 | Neuregulin-2 | Neuregulin-2 | [102] | |
| NSUN2 | NSUN2 | tRNA (cytosine(34)-C(5))-methyltransferase | [25] | |
| NTMT1 | NTMT1 | N-terminal Xaa-Pro-Lys N-methyltransferase 1 | [25] | |
| NUDC | NUDC | Nuclear migration protein nudC | [25] | |
| NUFIP1 | NUFIP | NUFIP1, FMR1 Interacting Protein 1 | [105] | |
| NUFIP2 | NUFIP2 | Nuclear fragile X mental retardation-interacting protein 2 | [25][44][45][89][60] | |
| NUPL2 | NUPL2 | Nucleoporin Like 2 | [137] | |
| NUP153 | NUP153 | Nucleoporin 153 | [44] | |
| NUP205 | NUP205 | Nuclear pore complex protein Nup205 | [25][137] | |
| NUP210 | NUP210/GP210 | Nucleoporin 210 | [137] | |
| NUP214 | NUP214 | Nucleoporin 214 | [137] | |
| NUP50 | NUP50 | Nucleoporin 50 | [137] | |
| NUP58 | NUP58/NUPL1 | Nucleoporin 58 | [137] | |
| NUP85 | NUP85 | Nucleoporin 85 | [137] | |
| NUP88 | NUP88 | Nucleoporin 88 | [137] | |
| NUP98 | NUP98/NUP96 | Nuclear pore complex protein Nup98-Nup96 | [25][137][60] | |
| OASL | OASL/OASL1 | 2'-5'-Oligoadenylate Synthetase Like | [175] | |
| OAS1 | OAS | 2′–5′ oligoadenylate synthetase | [87] | |
| OAS2 | OAS2 | 2'-5'-Oligoadenylate Synthetase 2 | [99] | |
| OGFOD1 | TPA1 | 2-Oxoglutarate And Iron Dependent Oxygenase Domain Containing 1 | [176] | |
| OGG1 | OGG1 | 8-Oxoguanine DNA Glycosylase | [177] | |
| OSBPL9 | Oxysterol Binding Protein Like 9 | Oxysterol Binding Protein Like 9 | [44] | |
| OTUD4 | OTUD4/HIN1 | OTU Deubiquitinase 4 | [44][45][178] | |
| P4HB | Prolyl 4-Hydroxylase Subunit Beta | Prolyl 4-Hydroxylase Subunit Beta | [44] | |
| PABPC1 | PABP1 | Poly(A) Binding Protein Cytoplasmic 1 | [25][44][45][148][112][55][120][70][105][134] | |
| PABPC4 | PABPC4 | Polyadenylate-binding protein 4 | [25][44][45] | |
| PAK4 | PAK4 | Serine/threonine-protein kinase PAK 4 | [25][44] | |
| PALLD | Palladin | Palladin | [25] | |
| PARG | PARG/PARG99/PARG102 | Poly(ADP-Ribose) Glycohydrolase | [179] | |
| PARK7 | PARK7/DJ-1 | Parkinsonism Associated Deglycase | [180] | yes[180] |
| PARN | PARN/DAN | Poly(A)-Specific Ribonuclease | [44] | |
| PARP12 | PARP-12/ARTD12 | Poly(ADP-Ribose) Polymerase Family Member 12 | [45][179][181] | |
| PARP14 | PARP-14 | Poly(ADP-Ribose) Polymerase Family Member 14 | [179] | |
| PARP15 | PARP-15 | Poly(ADP-Ribose) Polymerase Family Member 15 | [179] | |
| PATL1 | PATL1 | PAT1 Homolog 1, Processing Body mRNA Decay Factor | [44][45] | yes[45] |
| PAWR | PAWR | PRKC apoptosis WT1 regulator protein | [25] | |
| PCBP1 | PCBP1/HNRNPE1 | Poly(RC) Binding Protein 1 | [44][45] | |
| PCBP2 | PCBP2/HNRNPE2 | Poly(RC) Binding Protein 2 | [25][44][45][77] | |
| PCNA | PCNA | Proliferating cell nuclear antigen | [25] | |
| PDAP1 | PDAP1 | PDGFA Associated Protein 1 | [44] | |
| PDCD4 | PDCD4 | Programmed Cell Death 4 | [182] | |
| PDCD6IP | PDCD6IP | Programmed cell death 6-interacting protein | [25] | |
| PDIA3 | PDIA3 | Protein Disulfide Isomerase Family A Member 3 | [44] | |
| PDLIM1 | PDLIM1 | PDZ and LIM domain protein 1 | [25] | |
| PDLIM4 | PDLIM4 | PDZ and LIM domain protein 4 | [25] | |
| PDLIM5 | PDLIM5 | PDZ and LIM domain protein 5 | [25] | |
| PDS5B | PDS5B | Sister chromatid cohesion protein PDS5 homolog B | [25] | |
| PEF1 | PEF1 | Penta-EF-Hand Domain Containing 1 | [44] | |
| PEG10 | PEG10 | Paternally Expressed 10 | [45] | |
| PELO | PELO | Protein pelota homolog | [25] | |
| PEPD | Peptidase D | Peptidase D | [44] | |
| PEX11B | PEX11B | Peroxisomal Biogenesis Factor 11 Beta | [44] | |
| PFDN4 | PFDN4 | Prefoldin subunit 4 | [25] | |
| PFN1 | Profilin 1 | Profilin 1 | [25][59] | |
| PFN2 | Profilin 2 | Profilin 2 | [25][59] | |
| PGAM5 | PGAM5 | Serine/threonine-protein phosphatase PGAM5, mitochondrial | [25] | |
| PGP | PGP/G3PP | Phosphoglycolate Phosphatase | [44] | |
| PHB2 | Prohibitin 2 | Prohibitin 2 | [22] | |
| PHLDB2 | PHLDB2 | Pleckstrin homology-like domain family B member 2 | [25] | |
| PKP1 | Plakophilin 1 | Plakophilin 1 | [129] | |
| PKP2 | Plakophilin 2 | Plakophilin 2 | [25] | |
| PKP3 | Plakophilin 3 | Plakophilin 3 | [129] | |
| PNPT1 | PNPase I | Polyribonucleotide Nucleotidyltransferase 1 | [44] | |
| POLR2B | POLR2B | DNA-directed RNA polymerase | [25][60] | |
| POM121 | POM121 | POM121 Transmembrane Nucleoporin | [137] | |
| POP7 | RPP20 | POP7 Homolog, Ribonuclease P/MRP Subunit | [131] | |
| PPME1 | PPME1 | Protein phosphatase methylesterase 1 | [25] | |
| PPP1R8 | PPP1R8 | Protein Phosphatase 1 Regulatory Subunit 8 | [44] | |
| PPP1R10 | PPP1R10 | Serine/threonine-protein phosphatase 1 regulatory subunit 10 | [25][60] | |
| PPP1R18 | PPP1R18 | Phostensin | [25] | |
| PPP2R1A | PPP2R1A | Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform | [25][60] | |
| PPP2R1B | PPP2R1B | Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A beta isoform | [44] | |
| PQBP1 | PQBP-1 | Polyglutamine Binding Protein 1 | [183] | |
| PRDX1 | PRDX1 | Peroxiredoxin-1 | [25][44] | |
| PRDX6 | PRDX6 | Peroxiredoxin-6 | [25] | |
| PRKAA2 | AMPK-a2 | Protein Kinase AMP-Activated Catalytic Subunit Alpha 2 | [21] | |
| PRKCA | PKC-ɑ | Protein Kinase C Alpha | [134] | |
| PRKRA | PACT | Protein Activator Of Interferon Induced Protein Kinase EIF2AK2 | [25][54] | |
| PRMT1 | PRMT1 | Protein arginine N-methyltransferase 1 | [25] | |
| PRMT5 | PRMT5 | Protein arginine N-methyltransferase 5 | [25] | |
| PRRC2A | PRRC2A | Proline Rich Coiled-Coil 2A | [25][44][45] | |
| PRRC2B | PRRC2B | Proline Rich Coiled-Coil 2B | [44][45] | |
| PRRC2C | PRRC2C | Proline Rich Coiled-Coil 2C | [25][44][45][60] | |
| PSMD2 | PSMD2 | 26S proteasome non-ATPase regulatory subunit 2 | [25][184] | |
| PSPC1 | PSP1 | Paraspeckle Component 1 | [44] | |
| PTBP1 | PTBP1 | Polypyrimidine tract-binding protein 1 | [44] | |
| PTBP3 | PTBP3 | Polypyrimidine tract-binding protein 3 | [25][44][45] | |
| PTGES3 | PTGES3 | Prostaglandin E synthase 3 | [25] | |
| PTK2 | FAK | Protein Tyrosine Kinase 2 | [138] | |
| PUM1 | Pumilio-1 | Pumilio homolog 1 | [25][44][45] | yes[35] |
| PUM2 | Pumilio-2 | Pumilio RNA Binding Family Member 2 | [44][45][70] | |
| PURA | PURA | Transcriptional activator protein Pur-alpha | [25][44][124][126] | |
| PURB | PURB | Transcriptional activator protein Pur-beta | [25][44] | |
| PWP1 | PWP1 | PWP1 Homolog, Endonuclein | [44] | |
| PXDNL | PMR1 | Peroxidasin Like | [185] | |
| PYCR1 | PYCR1 | Pyrroline-5-carboxylate reductase | [25] | |
| QKI | QKI/HQK | QKI, KH Domain Containing RNA Binding | [44] | |
| R3HDM1 | R3HDM1 | R3H Domain Containing 1 | [44][45] | |
| R3HDM2 | R3HDM2 | R3H Domain Containing 2 | [45] | |
| RAB1A | RAB1A | Ras-related protein Rab-1A | [25][60] | |
| RACGAP1 | RACGAP1 | Rac GTPase-activating protein 1 | [25] | |
| RACK1 | RACK1 | Receptor For Activated C Kinase 1 | [22][110][186] | |
| RAD21 | RAD21 | Double-strand-break repair protein rad21 homolog | [25] | |
| RAE1 | RAE1 | Ribonucleic Acid Export 1 | [137] | |
| RAN | RAN | RAN, Member RAS Oncogene Family | [158][137] | |
| RANBP1 | RANBP1 | Ran-specific GTPase-activating protein | [25] | |
| RANBP2 | RANBP2/NUP358 | RAN Binding Protein 2 | [137] | |
| RBBP4 | RBBP4 | Histone-binding protein RBBP4 | [25] | |
| RBFOX1 | RBFOX1 | RNA binding protein fox-1 homolog | [25][187][188] | yes[188] |
| RBFOX2 | RBFOX2 | RNA binding protein fox-1 homolog 2 | [187] | |
| RBFOX3 | RBFOX3 | RNA binding protein fox-1 homolog 3 | [187] | |
| RBM12B | RBM12B | RNA-binding protein 12B | [25] | |
| RBM15 | RBM15 | RNA-binding protein 15 | [44] | |
| RBM17 | RBM17 | RNA-binding protein 17 | [44] | |
| RBM25 | RBM25 | RNA-binding protein 25 | [44] | |
| RBM26 | RBM26 | RNA-binding protein 26 | [25] | |
| RBM3 | RBM3 | RNA-binding protein 3 | [44] | |
| RBM38 | RBM38 | RNA-binding protein 38 | [44] | |
| RBM4 | RBM4 | RNA Binding Motif Protein 4 | [44][189] | |
| RBM4B | RBM4B | RNA Binding Motif Protein 4B | [44] | |
| RBM42 | RBM42 | RNA Binding Motif Protein 42 | [147] | |
| RBM45 | RBM45 | RNA Binding Motif Protein 45 | [190][191] | |
| RBM47 | RBM47 | RNA Binding Motif Protein 47 | [45] | |
| RBMS1 | RBMS1 | RNA-binding motif, single-stranded-interacting protein 1 | [25][44][45] | |
| RBMS2 | RBMS2 | RNA-binding motif, single-stranded-interacting protein 2 | [25][44][45] | |
| RBMX | RBMX | RNA Binding Motif Protein, X-Linked | [45] | |
| RBPMS | RBPMS | RNA-binding protein with multiple splicing | [192] | |
| RC3H1 | Roquin-1 | Ring Finger And CCCH-Type Domains 1 | [44][45][193] | |
| RC3H2 | MNAB | Ring Finger And CCCH-Type Domains 2 | [45][193] | |
| RCC1 | RCC1 | Regulator of chromosome condensation | [25] | |
| RCC2 | RCC2 | Protein RCC2 | [25] | |
| RECQL | RECQL1 | RecQ Like Helicase | [44] | |
| RFC3 | RFC3 | Replication factor C subunit 3 | [25] | |
| RFC4 | RFC4 | Replication factor C subunit 4 | [25] | |
| RGPD3 | RGPD3 | RanBP2-like and GRIP domain-containing protein 3 | [25] | |
| RHOA | RhoA | Ras Homolog Family Member A | [23] | |
| RNASEL | RNAse L | Ribonuclease L | [87][68] | |
| RNF214 | RNF214 | RING finger protein 214 | [25][44] | |
| RNF219 | RNF219 | RING finger protein 219 | [45] | yes[45] |
| RNF25 | RNF25 | Ring Finger Protein 25 | [44] | |
| RNH1 | RNH1 | Ribonuclease inhibitor | [25][53] | |
| ROCK1 | ROCK1 | Rho Associated Coiled-Coil Containing Protein Kinase 1 | [23] | |
| RPS19 | Ribosomal Protein S19 | Ribosomal Protein S19 | [100] | |
| RPS3 | 40S Ribosomal Protein S3 | 40S Ribosomal Protein S3 | [98][100] | yes[35] |
| RPS6 | Ribosomal Protein S6 | Ribosomal Protein S6 | [67][98][4][105][172] | |
| RPS11 | Ribosomal Protein S11 | Ribosomal Protein S11 | [44] | |
| RPS24 | Ribosomal Protein S24 | Ribosomal Protein S24 | [44] | |
| RPS6KA3 | RSK2 | Ribosomal Protein S6 Kinase A3 | [194] | |
| RPS6KB1 | S6K1 | Ribosomal Protein S6 Kinase B1 | [172] | |
| RPS6KB2 | S6K2 | Ribosomal Protein S6 Kinase B2 | [172] | |
| RPTOR | RAPTOR | Regulatory Associated Protein of mTOR Complex 1 | [88][96][172] | |
| RSL1D1 | RSL1D1 | Ribosomal L1 domain-containing protein 1 | [25] | |
| RTCB | RTCB | tRNA-splicing ligase RtcB homolog, formerly C22orf28 | [25][44] | |
| RTRAF | RTRAF (formerly C14orf166) | RNA Transcription, Translation And Transport Factor | [44] | |
| S100A7A | S100A7A | Protein S100-A7A | [25] | |
| S100A9 | S100A9 | Protein S100-A9 | [25] | yes[35] |
| SAFB2 | SAFB2 | Scaffold attachment factor B2 | [25][44] | yes[35] |
| SAMD4A | SMAUG1 | Sterile Alpha Motif Domain Containing 4A | [195] | |
| SAMD4B | SMAUG2 | Sterile Alpha Motif Domain Containing 4B | [44] | |
| SCAPER | SCAPER | S-Phase Cyclin A Associated Protein In The ER | [45] | |
| SEC24C | SEC24C | Protein transport protein Sec24C | [25][44] | |
| SECISBP2 | SECIS Binding Protein 2 | SECIS Binding Protein 2 | [44][45] | |
| SERBP1 | PAI-RBP1/SERBP1 | SERPINE1 mRNA Binding Protein 1 | [49][196][84] | |
| SERPINE1 | PAI-1/Serpin E1 | Serpine Family E Member 1 | [197] | |
| SF1 | SF1 | Splicing Factor 1 | [44] | |
| SFN | SFN | 14-3-3 protein sigma | [25] | |
| SFPQ | PSF | Splicing Factor Proline And Glutamine Rich | [25][174] | |
| SFRS3 | SFRS3 | Serine/arginine-rich splicing factor 3 | [25] | |
| SIPA1L1 | SIPA1L1 | Signal-induced proliferation-associated 1-like protein 1 | [25] | |
| SIRT6 | Sirtuin 6 | Sirtuin 6 | [198] | |
| SLBP | Stem-Loop Binding Protein | Stem-Loop Binding Protein | [44] | |
| SMAP2 | SMAP2 | Small ArfGAP2 | [45] | |
| SMARCA1 | SMARCA1/SNF2L1 | Probable global transcription activator SNF2L1 | [25] | |
| SMC4 | SMC4 | Structural maintenance of chromosomes protein | [25] | |
| SMG1 | SMG-1 | SMG1, Nonsense Mediated mRNA Decay Associated PI3K Related Kinase | [195][199] | |
| SMG6 | SMG6 | SMG6, Nonsense Mediated mRNA Decay Factor | [45] | |
| SMG7 | SMG7 | SMG7, Nonsense Mediated mRNA Decay Factor | [45] | yes[45] |
| SMN1 | Survival of Motor Neuron | Survival Of Motor Neuron 1, Telomeric | [131][200][201] | |
| SMU1 | SMU1 | WD40 repeat-containing protein SMU1 | [25] | |
| SMYD5 | SMYD5 | SMYD Family Member 5 | [44] | |
| SND1 | Tudor-SN | Staphylococcal Nuclease And Tudor Domain Containing 1 | [44][45][47][202] | |
| SNRPF | SNRPF | Small nuclear ribonucleoprotein F | [25] | |
| SNTB2 | SNTB2 | Beta-2-syntrophin | [25] | |
| SOGA3 | SOGA3 | SOGA Family Member 3 | [44] | |
| SORBS1 | SORBS1 | Sorbin and SH3 domain-containing protein 1 | [25] | |
| SORBS3 | Vinexin | Sorbin And SH3 Domain Containing 3 | [203] | |
| SOX3 | SOX3 | SRY-Box 3 | [44] | |
| SPAG5 | Astrin | Sperm Associated Antigen 5 | [88][172] | |
| SPATS2 | SPATS2/SPATA10/SCR59 | Spermatogenesis Associated Serine Rich 2 | [44] | |
| SPATS2L | SGNP | Spermatogenesis Associated Serine Rich 2 Like | [25][204] | |
| SPECC1L | SPECC1L | Cytospin-A | [25] | |
| SQSTM1 | SQSTM1/p62 | Sequestosome 1 | [63] | |
| SRI | SRI | Sorcin | [25][44] | |
| SRP68 | Signal Recognition Particle 68 | Signal Recognition Particle 68 | [44][48] | |
| SRP9 | SRP9 | Signal Recognition Particle 9 | [205] | |
| SRRT | SRRT | Serrate RNA effector molecule homolog | [25] | |
| SRSF1 | ASF/SF2 | Serine And Arginine Rich Splicing Factor 1 | [44][206] | |
| SRSF3 | SRp20 | Serine And Arginine Rich Splicing Factor 3 | [207][208][209][60] | |
| SRSF4 | SRSF4 | Serine/arginine-rich splicing factor 4 | [25] | |
| SRSF5 | SRSF5/SRP40 | Serine/arginine-rich splicing factor 5 | [44] | |
| SRSF7 | 9G8 | Serine And Arginine Rich Splicing Factor 7 | [49] | |
| SRSF9 | SRSF9/SRP30C | Serine/arginine-rich splicing factor 9 | [44] | |
| SS18L1 | SS18L1/CREST | SS18L1, nBAF Chromatin Remodeling Complex Subunit | [210] | |
| ST7 | ST7/FAM4A1/HELG/RAY1/TSG7 | Suppression Of Tumorigenicity 7 | [45] | yes[45] |
| STAT1 | STAT1 | Signal transducer and activator of transcription 1-alpha/beta | [25] | |
| STAU1 | Staufen 1 | Staufen Double-Stranded RNA Binding Protein 1 | [25][44][112][70][211] | |
| STAU2 | Staufen 2 | Staufen Double-Stranded RNA Binding Protein 2 | [25][44][45][112] | yes[35] |
| STIP1 | STIP1/HOP | Stress-induced-phosphoprotein 1 | [25][54] | |
| STRAP | STRAP | Serine-threonine kinase receptor-associated protein | [25][44] | |
| SUGP2 | SUGP2 | SURP and G-patch domain-containing protein 2 | [25] | |
| SUGT1 | SUGT1 | SGT1 Homolog, MIS12 Kinetochore Complex Assembly Cochaperone | [45] | |
| SUN1 | SUN1 | SUN domain-containing protein 1 | [25] | |
| SYCP3 | SYCP3 | Synaptonemal complex protein 3 | [25] | |
| SYK | SYK | Spleen Associated Tyrosine Kinase | [139] | |
| SYNCRIP | SYNCRIP | Heterogeneous nuclear ribonucleoprotein Q | [25][44][45][212] | yes[35] |
| TAGLN3 | Transgelin 3 | Transgelin 3 | [44] | |
| TAF15 | TAF15 | TATA-Box Binding Protein Associated Factor 15 | [25][44][117][118][122][60] | |
| TARDBP | TDP-43 | TAR DNA Binding Protein | [25][113][213][214][143][146][103][191][215][216] | |
| TBRG1 | TBRG1 | Transforming Growth Factor Beta Regulator 1 | [44] | |
| TCEA1 | TCEA1 | Transcription elongation factor A protein 1 | [25] | |
| TCP1 | TCP1 | T-complex protein 1 subunit alpha | [25] | |
| TDRD3 | Tudor Domain Containing 3 | Tudor Domain Containing 3 | [44][45][84][217][218][219] | |
| TDRD7 | Tudor Domain Containing 7 | Tudor Domain Containing 7 | [45] | |
| TERT | TERT | Telomerase Reverse Transcriptase | [220] | |
| THOC2 | THOC2 | THO Complex 2 | [137] | |
| THRAP3 | THRAP3 | Thyroid Hormone Receptor Associated Protein 3 | [44] | |
| TIA1 | TIA-1 | TIA1 Cytotoxic Granule Associated RNA Binding Protein | [4][25][44][49][56][34][70][80][95][121][132][142][148][200][215][221][60] | |
| TIAL1 | TIAR | TIA1 Cytotoxic Granule Associated RNA Binding Protein Like 1 | [25][44][45][70][105][112][113][148][190][200][210] | |
| TMEM131 | TMEM131 | Transmembrane Protein 131 | [45] | yes[45] |
| TMOD3 | TMOD3 | Tropomodulin-3 | [25] | |
| TNKS | PARP-5a | Tankyrase | [179] | |
| TNKS1BP1 | TNKS1BP1 | 182 kDa tankyrase-1-binding protein | [25][45] | yes[45] |
| TNPO1 | Transportin-1 | Transportin-1/Karyopherin (Importin) Beta 2 | [25][44][137][222][223] | |
| TNPO2 | Transportin-2 | Transportin-2 | [25][45] | |
| TNRC6A | TNRC6A | Trinucleotide repeat-containing gene 6A protein | [44][45] | yes[45] |
| TNRC6B | TNRC6B | Trinucleotide repeat-containing gene 6B protein | [25][44][45] | yes[45] |
| TNRC6C | TNRC6C | Trinucleotide repeat-containing gene 6C protein | [44][45] | yes[45] |
| TOMM34 | TOMM34 | Mitochondrial import receptor subunit TOM34 | [25] | |
| TOP3B | Topoisomerase (DNA) III Beta | Topoisomerase (DNA) III Beta | [45][218][224] | |
| TPM1 | TPM1 | Tropomyosin alpha-1 chain | [25] | |
| TPM2 | TPM2 | Tropomyosin beta chain | [25] | |
| TPR | TPR | Translocated Promoter Region, Nuclear Basket Protein | [137] | |
| TRA2B | TRA2B | Transformer 2 Beta Homolog | [45] | |
| TRAF2 | TRAF2 | TNF Receptor Associated Factor 2 | [109] | |
| TRDMT1 | DNMT2 | tRNA Aspartic Acid Methyltransferase 1 | [225] | |
| TRIM21 | TRIM21 | E3 ubiquitin-protein ligase TRIM21 | [25] | |
| TRIM25 | TRIM25 | E3 ubiquitin/ISG15 ligase TRIM25 | [25][44][60] | |
| TRIM56 | TRIM56 | E3 ubiquitin-protein ligase TRIM56 | [25][45][60] | |
| TRIM71 | TRIM71 | E3 ubiquitin-protein ligase TRIM71 | [44] | |
| TRIP6 | TRIP6 | Thyroid receptor-interacting protein 6 | [25][44] | |
| TROVE2 | RORNP | TROVE Domain Family Member 2 | [44] | |
| TTC17 | TTC17 | Tetratricopeptide Repeat Domain 17 | [45] | yes[45] |
| TUBA1C | TUBA1C | Tubulin alpha-1C chain | [25] | |
| TUBA3C | TUBA3C | Tubulin alpha-3C/D chain | [25] | |
| TUBA4A | TUBA4A | Tubulin alpha-4A chain | [25] | |
| TUBB3 | TUBB3 | Tubulin beta-3 chain | [25] | |
| TUBB8 | TUBB8 | Tubulin beta-8 chain | [25] | |
| TUFM | TUFM | Elongation factor Tu, mitochondrial | [25] | |
| TXN | TXN | Thioredoxin | [25] | |
| TXNDC17 | TXNDC17 | Thioredoxin Domain Containing 17 | [44] | |
| U2AF1 | U2AF1 | Splicing factor U2AF 35 kDa subunit | [25] | |
| UBA1 | UBA1 | Ubiquitin-like modifier-activating enzyme 1 | [25] | |
| UBAP2 | UBAP2 | Ubiquitin-associated protein 2 | [25][44][45][60] | |
| UBAP2L | UBAP2L | Ubiquitin-associated protein 2-like | [25][44][45][226][227][60] | |
| UBB | Ubiquitin | Ubiquitin | [114][132] | |
| UBL5 | Ubiquitin Like 5 | Ubiquitin Like 5 | [44] | |
| UBQLN2 | Ubiquilin 2 | Ubiquilin 2 | [228] | |
| ULK1 | ULK1 | Unc-51 Like Autophagy Activating Kinase 1 | [229] | |
| ULK2 | ULK2 | Unc-51 Like Autophagy Activating Kinase 2 | [229] | |
| UPF1 | UPF1 | UPF1, RNA Helicase and ATPase | [25][44][45][199][60] | yes[35] |
| UPF2 | UPF2 | UPF2, RNA Helicase and ATPase | [199] | |
| UPF3B | UPF3B | UPF3B, Regulator of Nonsense Mediated mRNA Decay | [44] | |
| USP10 | USP10 | Ubiquitin Specific Peptidase 10 | [25][44][45][67][34][186][60] | |
| USP11 | USP11 | Ubiquitin Specific Peptidase 11 | [44] | |
| USP13 | USP13 | Ubiquitin Specific Peptidase 13 | [230] | |
| USP5 | USP5 | Ubiquitin carboxyl-terminal hydrolase 5 | [25][230] | |
| USP9X | USP9X | Ubiquitin Specific Peptidase 9, X-Linked | [219] | |
| UTP18 | UTP18 | UTP18, Small Subunit Processome Component | [44] | |
| VASP | VASP | Vasodilator-stimulated phosphoprotein | [25] | |
| VBP1 | VBP1 | VHL Binding Protein 1 | [44] | |
| VCP | VCP | Valosin Containing Protein | [25][231][184][229] | |
| WBP2 | WBP2 | WW Domain Binding Protein 2 | [44] | |
| WDR47 | WDR47 | WD Repeat Domain 47 | [44] | |
| WDR62 | WDR62 | WD Repeat Domain 62 | [167] | |
| XPO1 | XPO1/CRM1 | Exportin 1 | [137] | |
| XRN1 | XRN1 | 5'-3' Exoribonuclease 1 | [36][44][45] | yes[36][45] |
| XRN2 | XRN2 | 5'-3' Exoribonuclease 2 | [44] | |
| YARS | YARS | Tyrosine—tRNA ligase, cytoplasmic | [25] | |
| YBX1 | YB-1 | Y-Box Binding Protein 1 | [25][44][49][48][82][94][232] | |
| YBX3 | YBX3/ZONAB | Y-box-binding protein 3 | [25][44][45] | |
| YES1 | YES1 | Tyrosine-protein kinase Yes | [25] | |
| YLPM1 | YLPM1 | YLP Motif Containing 1 | [44] | |
| YTHDF1 | YTHDF1 | YTH domain family protein 1 | [25][44][45][233][234] | |
| YTHDF2 | YTHDF2 | YTH domain family protein 2 | [25][44][45][233][234] | yes[233][234] |
| YTHDF3 | YTHDF3 | YTH domain family protein 3 | [25][32][44][45][233][234] | |
| YWHAB | 14-3-3 | Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Beta | [25][168] | |
| YWHAH | 14-3-3 | 14-3-3 protein eta | [25] | |
| YWHAQ | 14-3-3 | 14-3-3 protein theta | [25] | |
| ZBP1 | ZBP1 | Z-DNA Binding Protein 1 | [235][236] | |
| ZCCHC11 | ZCCHC11 | Zinc finger CCCH domain-containing protein 11 | [45] | |
| ZCCHC14 | ZCCHC14 | Zinc finger CCCH domain-containing protein 14 | [45] | |
| ZC3H11A | ZC3H11A | Zinc finger CCCH domain-containing protein 11a | [44] | |
| ZC3H14 | ZC3H14 | Zinc finger CCCH domain-containing protein 14 | [25] | |
| ZCCHC2 | ZCCHC2 | Zinc finger CCCH domain-containing protein 2 | [45] | |
| ZCCHC3 | ZCCHC3 | Zinc finger CCCH domain-containing protein 3 | [45] | |
| ZC3H7A | ZC3H7A | Zinc finger CCCH domain-containing protein 7A | [25] | |
| ZC3H7B | ZC3H7B | Zinc finger CCCH domain-containing protein 7B | [25][44] | |
| ZC3HAV1 | PARP-13.1/PARP-13.2/ARTD13 | Zinc Finger CCCH-Type Containing, Antiviral 1 | [25][45][179] | yes[35] |
| ZFAND1 | ZFAND1 | Zinc Finger AN1-Type Containing 1 | [184] | |
| ZFP36 | TTP/TIS11 | ZFP36 Ring Finger Protein/Trisetrapolin | [36][44][167][237][238][239] | yes[36] |
| ZNF598 | ZNF598 | Zinc finger protein 598 | [45] | |
| ZNF638 | ZNF638 | Zinc finger protein 638 | [25] |
References
- Gutierrez-Beltran E, Moschou PN, Smertenko AP, Bozhkov PV (March 2015). "Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis". The Plant Cell. 27 (3): 926–943. doi:10.1105/tpc.114.134494. PMC 4558657. PMID 25736060.
- Hirose, Tetsuro; Ninomiya, Kensuke; Nakagawa, Shinichi; Yamazaki, Tomohiro (2022-11-23). "A guide to membraneless organelles and their various roles in gene regulation". Nature Reviews Molecular Cell Biology. 24 (4): 288–304. doi:10.1038/s41580-022-00558-8. ISSN 1471-0080. PMID 36424481. S2CID 253879916.
- Hirose, Tetsuro; Ninomiya, Kensuke; Nakagawa, Shinichi; Yamazaki, Tomohiro (April 2023). "A guide to membraneless organelles and their various roles in gene regulation". Nature Reviews Molecular Cell Biology. 24 (4): 288–304. doi:10.1038/s41580-022-00558-8. ISSN 1471-0080. PMID 36424481. S2CID 253879916.
- Kayali F, Montie HL, Rafols JA, DeGracia DJ (2005). "Prolonged translation arrest in reperfused hippocampal cornu Ammonis 1 is mediated by stress granules". Neuroscience. 134 (4): 1223–1245. doi:10.1016/j.neuroscience.2005.05.047. PMID 16055272. S2CID 15066267.
- Nover L, Scharf KD, Neumann D (March 1989). "Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs". Molecular and Cellular Biology. 9 (3): 1298–1308. doi:10.1128/mcb.9.3.1298. PMC 362722. PMID 2725500.
- Paul J. Anderson, Brigham and Women's Hospital
- Mollet S, Cougot N, Wilczynska A, Dautry F, Kress M, Bertrand E, Weil D (October 2008). "Translationally repressed mRNA transiently cycles through stress granules during stress". Molecular Biology of the Cell. 19 (10): 4469–4479. doi:10.1091/mbc.E08-05-0499. PMC 2555929. PMID 18632980.
- Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R (November 2017). "The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules". Molecular Cell. 68 (4): 808–820.e5. doi:10.1016/j.molcel.2017.10.015. PMC 5728175. PMID 29129640.
- Khong A, Parker R (December 2018). "mRNP architecture in translating and stress conditions reveals an ordered pathway of mRNP compaction". The Journal of Cell Biology. 217 (12): 4124–4140. doi:10.1083/jcb.201806183. PMC 6279387. PMID 30322972.
- Khong A, Jain S, Matheny T, Wheeler JR, Parker R (March 2018). "Isolation of mammalian stress granule cores for RNA-Seq analysis". Methods. 137: 49–54. doi:10.1016/j.ymeth.2017.11.012. PMC 5866748. PMID 29196162.
- Forreiter C, Kirschner M, Nover L (December 1997). "Stable transformation of an Arabidopsis cell suspension culture with firefly luciferase providing a cellular system for analysis of chaperone activity in vivo". The Plant Cell. 9 (12): 2171–2181. doi:10.1105/tpc.9.12.2171. PMC 157066. PMID 9437862.
- Löw D, Brändle K, Nover L, Forreiter C (September 2000). "Cytosolic heat-stress proteins Hsp17.7 class I and Hsp17.3 class II of tomato act as molecular chaperones in vivo". Planta. 211 (4): 575–582. doi:10.1007/s004250000315. PMID 11030557. S2CID 9646838.
- Stuger R, Ranostaj S, Materna T, Forreiter C (May 1999). "Messenger RNA-binding properties of nonpolysomal ribonucleoproteins from heat-stressed tomato cells". Plant Physiology. 120 (1): 23–32. doi:10.1104/pp.120.1.23. PMC 59255. PMID 10318680.
- Schmid HP, Akhayat O, Martins De Sa C, Puvion F, Koehler K, Scherrer K (January 1984). "The prosome: an ubiquitous morphologically distinct RNP particle associated with repressed mRNPs and containing specific ScRNA and a characteristic set of proteins". The EMBO Journal. 3 (1): 29–34. doi:10.1002/j.1460-2075.1984.tb01757.x. PMC 557293. PMID 6200323.
- Aulas A, Lyons SM, Fay MM, Anderson P, Ivanov P (November 2018). "Nitric oxide triggers the assembly of "type II" stress granules linked to decreased cell viability". Cell Death & Disease. 9 (11): 1129. doi:10.1038/s41419-018-1173-x. PMC 6234215. PMID 30425239.
- Berchtold D, Battich N, Pelkmans L (December 2018). "A Systems-Level Study Reveals Regulators of Membrane-less Organelles in Human Cells". Molecular Cell. 72 (6): 1035–1049.e5. doi:10.1016/j.molcel.2018.10.036. PMID 30503769.
- Aulas A, Fay MM, Lyons SM, Achorn CA, Kedersha N, Anderson P, Ivanov P (March 2017). "Stress-specific differences in assembly and composition of stress granules and related foci". Journal of Cell Science. 130 (5): 927–937. doi:10.1242/jcs.199240. PMC 5358336. PMID 28096475.
- Qifti, Androniqi; Jackson, Lela; Singla, Ashima; Garwain, Osama; Scarlata, Suzanne (2021-10-19). "Stimulation of phospholipase Cβ1 by Gα q promotes the assembly of stress granule proteins". Science Signaling. 14 (705). doi:10.1126/scisignal.aav1012. ISSN 1945-0877.
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P (December 2004). "Stress granule assembly is mediated by prion-like aggregation of TIA-1". Molecular Biology of the Cell. 15 (12): 5383–5398. doi:10.1091/mbc.E04-08-0715. PMC 532018. PMID 15371533.
- Ivanov PA, Chudinova EM, Nadezhdina ES (November 2003). "Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation". Experimental Cell Research. 290 (2): 227–233. doi:10.1016/S0014-4827(03)00290-8. PMID 14567982.
- Mahboubi H, Barisé R, Stochaj U (July 2015). "5'-AMP-activated protein kinase alpha regulates stress granule biogenesis". Biochimica et Biophysica Acta. 1853 (7): 1725–1737. doi:10.1016/j.bbamcr.2015.03.015. PMID 25840010.
- Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P (October 2008). "A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly". Nature Cell Biology. 10 (10): 1224–1231. doi:10.1038/ncb1783. PMC 4318256. PMID 18794846.
- Tsai NP, Wei LN (April 2010). "RhoA/ROCK1 signaling regulates stress granule formation and apoptosis". Cellular Signalling. 22 (4): 668–675. doi:10.1016/j.cellsig.2009.12.001. PMC 2815184. PMID 20004716.
- Van Treeck B, Protter DS, Matheny T, Khong A, Link CD, Parker R (March 2018). "RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome". Proceedings of the National Academy of Sciences of the United States of America. 115 (11): 2734–2739. Bibcode:2018PNAS..115.2734V. doi:10.1073/pnas.1800038115. PMC 5856561. PMID 29483269.
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R (January 2016). "ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure". Cell. 164 (3): 487–498. doi:10.1016/j.cell.2015.12.038. PMC 4733397. PMID 26777405.
- Chalupníková K, Lattmann S, Selak N, Iwamoto F, Fujiki Y, Nagamine Y (December 2008). "Recruitment of the RNA helicase RHAU to stress granules via a unique RNA-binding domain". The Journal of Biological Chemistry. 283 (50): 35186–35198. doi:10.1074/jbc.M804857200. PMC 3259895. PMID 18854321.
- Hilliker A, Gao Z, Jankowsky E, Parker R (September 2011). "The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex". Molecular Cell. 43 (6): 962–972. doi:10.1016/j.molcel.2011.08.008. PMC 3268518. PMID 21925384.
- Epling LB, Grace CR, Lowe BR, Partridge JF, Enemark EJ (May 2015). "Cancer-associated mutants of RNA helicase DDX3X are defective in RNA-stimulated ATP hydrolysis". Journal of Molecular Biology. 427 (9): 1779–1796. doi:10.1016/j.jmb.2015.02.015. PMC 4402148. PMID 25724843.
- Valentin-Vega YA, Wang YD, Parker M, Patmore DM, Kanagaraj A, Moore J, et al. (May 2016). "Cancer-associated DDX3X mutations drive stress granule assembly and impair global translation". Scientific Reports. 6 (1): 25996. Bibcode:2016NatSR...625996V. doi:10.1038/srep25996. PMC 4867597. PMID 27180681.
- Van Treeck B, Parker R (August 2018). "Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies". Cell. 174 (4): 791–802. doi:10.1016/j.cell.2018.07.023. PMC 6200146. PMID 30096311.
- Adivarahan S, Livingston N, Nicholson B, Rahman S, Wu B, Rissland OS, Zenklusen D (November 2018). "Spatial Organization of Single mRNPs at Different Stages of the Gene Expression Pathway". Molecular Cell. 72 (4): 727–738.e5. doi:10.1016/j.molcel.2018.10.010. PMC 6592633. PMID 30415950.
- Anders M, Chelysheva I, Goebel I, Trenkner T, Zhou J, Mao Y, et al. (August 2018). "Dynamic m6A methylation facilitates mRNA triaging to stress granules". Life Science Alliance. 1 (4): e201800113. doi:10.26508/lsa.201800113. PMC 6238392. PMID 30456371.
- Tauber D, Tauber G, Khong A, Van Treeck B, Pelletier J, Parker R (February 2020). "Modulation of RNA Condensation by the DEAD-Box Protein eIF4A". Cell. 180 (3): 411–426.e16. doi:10.1016/j.cell.2019.12.031. PMC 7194247. PMID 31928844.
- Aulas A, Caron G, Gkogkas CG, Mohamed NV, Destroismaisons L, Sonenberg N, et al. (April 2015). "G3BP1 promotes stress-induced RNA granule interactions to preserve polyadenylated mRNA". The Journal of Cell Biology. 209 (1): 73–84. doi:10.1083/jcb.201408092. PMC 4395486. PMID 25847539.
- Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, et al. (October 2017). "P-Body Purification Reveals the Condensation of Repressed mRNA Regulons". Molecular Cell. 68 (1): 144–157.e5. doi:10.1016/j.molcel.2017.09.003. PMID 28965817.
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, et al. (June 2005). "Stress granules and processing bodies are dynamically linked sites of mRNP remodeling". The Journal of Cell Biology. 169 (6): 871–884. doi:10.1083/jcb.200502088. PMC 2171635. PMID 15967811.
- Buchan JR, Muhlrad D, Parker R (November 2008). "P bodies promote stress granule assembly in Saccharomyces cerevisiae". The Journal of Cell Biology. 183 (3): 441–455. doi:10.1083/jcb.200807043. PMC 2575786. PMID 18981231.
- Figley MD (2015). Profilin 1, stress granules, and ALS pathogenesis (PhD). Stanford University.
- Aulas A, Vande Velde C (2015). "Alterations in stress granule dynamics driven by TDP-43 and FUS: a link to pathological inclusions in ALS?". Frontiers in Cellular Neuroscience. 9: 423. doi:10.3389/fncel.2015.00423. PMC 4615823. PMID 26557057.
- Youn JY, Dyakov BJ, Zhang J, Knight JD, Vernon RM, Forman-Kay JD, Gingras AC (October 2019). "Properties of Stress Granule and P-Body Proteomes". Molecular Cell. 76 (2): 286–294. doi:10.1016/j.molcel.2019.09.014. PMID 31626750.
- Aulas A, Fay MM, Szaflarski W, Kedersha N, Anderson P, Ivanov P (May 2017). "Methods to Classify Cytoplasmic Foci as Mammalian Stress Granules". Journal of Visualized Experiments (123). doi:10.3791/55656. PMC 5607937. PMID 28570526.
- Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R (September 2016). "Distinct stages in stress granule assembly and disassembly". eLife. 5. doi:10.7554/eLife.18413. PMC 5014549. PMID 27602576.
- Wheeler JR, Jain S, Khong A, Parker R (August 2017). "Isolation of yeast and mammalian stress granule cores". Methods. 126: 12–17. doi:10.1016/j.ymeth.2017.04.020. PMC 5924690. PMID 28457979.
- Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, et al. (January 2018). "Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules". Cell. 172 (3): 590–604.e13. doi:10.1016/j.cell.2017.12.032. PMC 5969999. PMID 29373831.
- Youn JY, Dunham WH, Hong SJ, Knight JD, Bashkurov M, Chen GI, et al. (February 2018). "High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies". Molecular Cell. 69 (3): 517–532.e11. doi:10.1016/j.molcel.2017.12.020. PMID 29395067.
- Marmor-Kollet H, Siany A, Kedersha N, Knafo N, Rivkin N, Danino YM, et al. (December 2020). "Spatiotemporal Proteomic Analysis of Stress Granule Disassembly Using APEX Reveals Regulation by SUMOylation and Links to ALS Pathogenesis". Molecular Cell. 80 (5): 876–891.e6. doi:10.1016/j.molcel.2020.10.032. PMC 7816607. PMID 33217318.
- Weissbach R, Scadden AD (March 2012). "Tudor-SN and ADAR1 are components of cytoplasmic stress granules". RNA. 18 (3): 462–471. doi:10.1261/rna.027656.111. PMC 3285934. PMID 22240577.
- Gallois-Montbrun S, Kramer B, Swanson CM, Byers H, Lynham S, Ward M, Malim MH (March 2007). "Antiviral protein APOBEC3G localizes to ribonucleoprotein complexes found in P bodies and stress granules". Journal of Virology. 81 (5): 2165–2178. doi:10.1128/JVI.02287-06. PMC 1865933. PMID 17166910.
- Goodier JL, Zhang L, Vetter MR, Kazazian HH (September 2007). "LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex". Molecular and Cellular Biology. 27 (18): 6469–6483. doi:10.1128/MCB.00332-07. PMC 2099616. PMID 17562864.
- Detzer A, Engel C, Wünsche W, Sczakiel G (April 2011). "Cell stress is related to re-localization of Argonaute 2 and to decreased RNA interference in human cells". Nucleic Acids Research. 39 (7): 2727–2741. doi:10.1093/nar/gkq1216. PMC 3074141. PMID 21148147.
- Lou Q, Hu Y, Ma Y, Dong Z (January 2019). "RNA interference may suppress stress granule formation by preventing argonaute 2 recruitment". American Journal of Physiology. Cell Physiology. 316 (1): C81–C91. doi:10.1152/ajpcell.00251.2018. PMC 6383145. PMID 30404558.
- Kolobova E, Efimov A, Kaverina I, Rishi AK, Schrader JW, Ham AJ, et al. (February 2009). "Microtubule-dependent association of AKAP350A and CCAR1 with RNA stress granules". Experimental Cell Research. 315 (3): 542–555. doi:10.1016/j.yexcr.2008.11.011. PMC 2788823. PMID 19073175.
- Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, et al. (September 2013). "Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival". Journal of Cell Science. 126 (Pt 18): 4308–4319. doi:10.1242/jcs.134551. PMC 3772394. PMID 23843625.
- Pare JM, Tahbaz N, López-Orozco J, LaPointe P, Lasko P, Hobman TC (July 2009). "Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies". Molecular Biology of the Cell. 20 (14): 3273–3284. doi:10.1091/mbc.E09-01-0082. PMC 2710822. PMID 19458189.
- Ralser M, Albrecht M, Nonhoff U, Lengauer T, Lehrach H, Krobitsch S (February 2005). "An integrative approach to gain insights into the cellular function of human ataxin-2". Journal of Molecular Biology. 346 (1): 203–214. doi:10.1016/j.jmb.2004.11.024. hdl:11858/00-001M-0000-0010-86DE-D. PMID 15663938.
- Nonhoff U, Ralser M, Welzel F, Piccini I, Balzereit D, Yaspo ML, et al. (April 2007). "Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules". Molecular Biology of the Cell. 18 (4): 1385–1396. doi:10.1091/mbc.E06-12-1120. PMC 1838996. PMID 17392519.
- Kaehler C, Isensee J, Nonhoff U, Terrey M, Hucho T, Lehrach H, Krobitsch S (2012). "Ataxin-2-like is a regulator of stress granules and processing bodies". PLOS ONE. 7 (11): e50134. Bibcode:2012PLoSO...750134K. doi:10.1371/journal.pone.0050134. PMC 3507954. PMID 23209657.
- Nihei Y, Ito D, Suzuki N (November 2012). "Roles of ataxin-2 in pathological cascades mediated by TAR DNA-binding protein 43 (TDP-43) and Fused in Sarcoma (FUS)". The Journal of Biological Chemistry. 287 (49): 41310–41323. doi:10.1074/jbc.M112.398099. PMC 3510829. PMID 23048034.
- Figley MD, Bieri G, Kolaitis RM, Taylor JP, Gitler AD (June 2014). "Profilin 1 associates with stress granules and ALS-linked mutations alter stress granule dynamics". The Journal of Neuroscience. 34 (24): 8083–8097. doi:10.1523/JNEUROSCI.0543-14.2014. PMC 4051967. PMID 24920614.
- Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, et al. (April 2020). "G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules". Cell. 181 (2): 325–345.e28. doi:10.1016/j.cell.2020.03.046. PMC 7448383. PMID 32302571.
- Kim B, Rhee K (2016). "BOULE, a Deleted in Azoospermia Homolog, Is Recruited to Stress Granules in the Mouse Male Germ Cells". PLOS ONE. 11 (9): e0163015. Bibcode:2016PLoSO..1163015K. doi:10.1371/journal.pone.0163015. PMC 5024984. PMID 27632217.
- Maharjan N, Künzli C, Buthey K, Saxena S (May 2017). "C9ORF72 Regulates Stress Granule Formation and Its Deficiency Impairs Stress Granule Assembly, Hypersensitizing Cells to Stress". Molecular Neurobiology. 54 (4): 3062–3077. doi:10.1007/s12035-016-9850-1. PMID 27037575. S2CID 27449387.
- Chitiprolu M, Jagow C, Tremblay V, Bondy-Chorney E, Paris G, Savard A, et al. (July 2018). "A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy". Nature Communications. 9 (1): 2794. Bibcode:2018NatCo...9.2794C. doi:10.1038/s41467-018-05273-7. PMC 6052026. PMID 30022074.
- Decca MB, Carpio MA, Bosc C, Galiano MR, Job D, Andrieux A, Hallak ME (March 2007). "Post-translational arginylation of calreticulin: a new isospecies of calreticulin component of stress granules". The Journal of Biological Chemistry. 282 (11): 8237–8245. doi:10.1074/jbc.M608559200. PMC 2702537. PMID 17197444.
- Solomon S, Xu Y, Wang B, David MD, Schubert P, Kennedy D, Schrader JW (March 2007). "Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs". Molecular and Cellular Biology. 27 (6): 2324–2342. doi:10.1128/MCB.02300-06. PMC 1820512. PMID 17210633.
- Ratovitski T, Chighladze E, Arbez N, Boronina T, Herbrich S, Cole RN, Ross CA (May 2012). "Huntingtin protein interactions altered by polyglutamine expansion as determined by quantitative proteomic analysis". Cell Cycle. 11 (10): 2006–2021. doi:10.4161/cc.20423. PMC 3359124. PMID 22580459.
- Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, et al. (March 2016). "G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits". The Journal of Cell Biology. 212 (7): 845–860. doi:10.1083/jcb.201508028. PMC 4810302. PMID 27022092.
- Reineke LC, Kedersha N, Langereis MA, van Kuppeveld FJ, Lloyd RE (March 2015). "Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1". mBio. 6 (2): e02486. doi:10.1128/mBio.02486-14. PMC 4453520. PMID 25784705.
- Baguet A, Degot S, Cougot N, Bertrand E, Chenard MP, Wendling C, et al. (August 2007). "The exon-junction-complex-component metastatic lymph node 51 functions in stress-granule assembly". Journal of Cell Science. 120 (Pt 16): 2774–2784. doi:10.1242/jcs.009225. PMID 17652158.
- Vessey JP, Vaccani A, Xie Y, Dahm R, Karra D, Kiebler MA, Macchi P (June 2006). "Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules". The Journal of Neuroscience. 26 (24): 6496–6508. doi:10.1523/JNEUROSCI.0649-06.2006. PMC 6674044. PMID 16775137.
- Moujalled D, James JL, Yang S, Zhang K, Duncan C, Moujalled DM, et al. (March 2015). "Phosphorylation of hnRNP K by cyclin-dependent kinase 2 controls cytosolic accumulation of TDP-43". Human Molecular Genetics. 24 (6): 1655–1669. doi:10.1093/hmg/ddu578. PMID 25410660.
- Fujimura K, Kano F, Murata M (February 2008). "Dual localization of the RNA binding protein CUGBP-1 to stress granule and perinucleolar compartment". Experimental Cell Research. 314 (3): 543–553. doi:10.1016/j.yexcr.2007.10.024. PMID 18164289.
- Fathinajafabadi A, Pérez-Jiménez E, Riera M, Knecht E, Gonzàlez-Duarte R (2014). "CERKL, a retinal disease gene, encodes an mRNA-binding protein that localizes in compact and untranslated mRNPs associated with microtubules". PLOS ONE. 9 (2): e87898. Bibcode:2014PLoSO...987898F. doi:10.1371/journal.pone.0087898. PMC 3912138. PMID 24498393.
- De Leeuw F, Zhang T, Wauquier C, Huez G, Kruys V, Gueydan C (December 2007). "The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor". Experimental Cell Research. 313 (20): 4130–4144. doi:10.1016/j.yexcr.2007.09.017. PMID 17967451.
- Rojas M, Farr GW, Fernandez CF, Lauden L, McCormack JC, Wolin SL (2012). "Yeast Gis2 and its human ortholog CNBP are novel components of stress-induced RNP granules". PLOS ONE. 7 (12): e52824. Bibcode:2012PLoSO...752824R. doi:10.1371/journal.pone.0052824. PMC 3528734. PMID 23285195.
- Cougot N, Babajko S, Séraphin B (April 2004). "Cytoplasmic foci are sites of mRNA decay in human cells". The Journal of Cell Biology. 165 (1): 31–40. doi:10.1083/jcb.200309008. PMC 2172085. PMID 15067023.
- Fujimura K, Kano F, Murata M (March 2008). "Identification of PCBP2, a facilitator of IRES-mediated translation, as a novel constituent of stress granules and processing bodies". RNA. 14 (3): 425–431. doi:10.1261/rna.780708. PMC 2248264. PMID 18174314.
- Wilczynska A, Aigueperse C, Kress M, Dautry F, Weil D (March 2005). "The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules". Journal of Cell Science. 118 (Pt 5): 981–992. doi:10.1242/jcs.01692. PMID 15731006.
- Reineke LC, Tsai WC, Jain A, Kaelber JT, Jung SY, Lloyd RE (February 2017). "Casein Kinase 2 Is Linked to Stress Granule Dynamics through Phosphorylation of the Stress Granule Nucleating Protein G3BP1". Molecular and Cellular Biology. 37 (4): e00596–16. doi:10.1128/MCB.00596-16. PMC 5288577. PMID 27920254.
- Kim JE, Ryu I, Kim WJ, Song OK, Ryu J, Kwon MY, et al. (January 2008). "Proline-rich transcript in brain protein induces stress granule formation". Molecular and Cellular Biology. 28 (2): 803–813. doi:10.1128/MCB.01226-07. PMC 2223406. PMID 17984221.
- Kim B, Cooke HJ, Rhee K (February 2012). "DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress". Development. 139 (3): 568–578. doi:10.1242/dev.075846. PMID 22223682.
- Onishi H, Kino Y, Morita T, Futai E, Sasagawa N, Ishiura S (July 2008). "MBNL1 associates with YB-1 in cytoplasmic stress granules". Journal of Neuroscience Research. 86 (9): 1994–2002. doi:10.1002/jnr.21655. PMID 18335541. S2CID 9431966.
- Yasuda-Inoue M, Kuroki M, Ariumi Y (November 2013). "DDX3 RNA helicase is required for HIV-1 Tat function". Biochemical and Biophysical Research Communications. 441 (3): 607–611. doi:10.1016/j.bbrc.2013.10.107. PMID 24183723.
- Goulet I, Boisvenue S, Mokas S, Mazroui R, Côté J (October 2008). "TDRD3, a novel Tudor domain-containing protein, localizes to cytoplasmic stress granules". Human Molecular Genetics. 17 (19): 3055–3074. doi:10.1093/hmg/ddn203. PMC 2536506. PMID 18632687.
- Valentin-Vega YA, Wang YD, Parker M, Patmore DM, Kanagaraj A, Moore J, et al. (May 2016). "Cancer-associated DDX3X mutations drive stress granule assembly and impair global translation". Scientific Reports. 6: 25996. Bibcode:2016NatSR...625996V. doi:10.1038/srep25996. PMC 4867597. PMID 27180681.
- Saito M, Hess D, Eglinger J, Fritsch AW, Kreysing M, Weinert BT, et al. (January 2019). "Acetylation of intrinsically disordered regions regulates phase separation". Nature Chemical Biology. 15 (1): 51–61. doi:10.1038/s41589-018-0180-7. PMID 30531905. S2CID 54471609.
- Onomoto K, Jogi M, Yoo JS, Narita R, Morimoto S, Takemura A, et al. (2012). "Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity". PLOS ONE. 7 (8): e43031. Bibcode:2012PLoSO...743031O. doi:10.1371/journal.pone.0043031. PMC 3418241. PMID 22912779.
- Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Kläsener K, Ruf S, et al. (August 2013). "Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells". Cell. 154 (4): 859–874. doi:10.1016/j.cell.2013.07.031. PMID 23953116.
- Bish R, Cuevas-Polo N, Cheng Z, Hambardzumyan D, Munschauer M, Landthaler M, Vogel C (July 2015). "Comprehensive Protein Interactome Analysis of a Key RNA Helicase: Detection of Novel Stress Granule Proteins". Biomolecules. 5 (3): 1441–1466. doi:10.3390/biom5031441. PMC 4598758. PMID 26184334.
- Salleron L, Magistrelli G, Mary C, Fischer N, Bairoch A, Lane L (December 2014). "DERA is the human deoxyribose phosphate aldolase and is involved in stress response". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1843 (12): 2913–2925. doi:10.1016/j.bbamcr.2014.09.007. PMID 25229427.
- Ogawa F, Kasai M, Akiyama T (December 2005). "A functional link between Disrupted-In-Schizophrenia 1 and the eukaryotic translation initiation factor 3". Biochemical and Biophysical Research Communications. 338 (2): 771–776. doi:10.1016/j.bbrc.2005.10.013. PMID 16243297.
- Belli V, Matrone N, Sagliocchi S, Incarnato R, Conte A, Pizzo E, et al. (December 2019). "A dynamic link between H/ACA snoRNP components and cytoplasmic stress granules". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1866 (12): 118529. doi:10.1016/j.bbamcr.2019.118529. PMID 31412274.
- Loschi M, Leishman CC, Berardone N, Boccaccio GL (November 2009). "Dynein and kinesin regulate stress-granule and P-body dynamics". Journal of Cell Science. 122 (Pt 21): 3973–3982. doi:10.1242/jcs.051383. PMC 2773196. PMID 19825938.
- Geng Q, Xhabija B, Knuckle C, Bonham CA, Vacratsis PO (January 2017). "The Atypical Dual Specificity Phosphatase hYVH1 Associates with Multiple Ribonucleoprotein Particles". The Journal of Biological Chemistry. 292 (2): 539–550. doi:10.1074/jbc.M116.715607. PMC 5241730. PMID 27856639.
- Tsai NP, Tsui YC, Wei LN (March 2009). "Dynein motor contributes to stress granule dynamics in primary neurons". Neuroscience. 159 (2): 647–656. doi:10.1016/j.neuroscience.2008.12.053. PMC 2650738. PMID 19171178.
- Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L (February 2013). "Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling". Cell. 152 (4): 791–805. doi:10.1016/j.cell.2013.01.033. PMID 23415227.
- Shigunov P, Sotelo-Silveira J, Stimamiglio MA, Kuligovski C, Irigoín F, Badano JL, et al. (July 2014). "Ribonomic analysis of human DZIP1 reveals its involvement in ribonucleoprotein complexes and stress granules". BMC Molecular Biology. 15: 12. doi:10.1186/1471-2199-15-12. PMC 4091656. PMID 24993635.
- Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP (February 2003). "Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes". American Journal of Physiology. Cell Physiology. 284 (2): C273–C284. doi:10.1152/ajpcell.00314.2002. PMID 12388085. S2CID 14681272.
- Reineke LC, Lloyd RE (March 2015). "The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses". Journal of Virology. 89 (5): 2575–2589. doi:10.1128/JVI.02791-14. PMC 4325707. PMID 25520508.
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P (January 2002). "Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules". Molecular Biology of the Cell. 13 (1): 195–210. doi:10.1091/mbc.01-05-0221. PMC 65082. PMID 11809833.
- Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P (April 2010). "eIF5A promotes translation elongation, polysome disassembly and stress granule assembly". PLOS ONE. 5 (4): e9942. Bibcode:2010PLoSO...5.9942L. doi:10.1371/journal.pone.0009942. PMC 2848580. PMID 20376341.
- Kim JA, Jayabalan AK, Kothandan VK, Mariappan R, Kee Y, Ohn T (August 2016). "Identification of Neuregulin-2 as a novel stress granule component". BMB Reports. 49 (8): 449–454. doi:10.5483/BMBRep.2016.49.8.090. PMC 5070733. PMID 27345716.
- Dammer EB, Fallini C, Gozal YM, Duong DM, Rossoll W, Xu P, et al. (2012). "Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination". PLOS ONE. 7 (6): e38658. Bibcode:2012PLoSO...738658D. doi:10.1371/journal.pone.0038658. PMC 3380899. PMID 22761693.
- Jongjitwimol J, Baldock RA, Morley SJ, Watts FZ (June 2016). "Sumoylation of eIF4A2 affects stress granule formation". Journal of Cell Science. 129 (12): 2407–2415. doi:10.1242/jcs.184614. PMC 4920252. PMID 27160682.
- Kim SH, Dong WK, Weiler IJ, Greenough WT (March 2006). "Fragile X mental retardation protein shifts between polyribosomes and stress granules after neuronal injury by arsenite stress or in vivo hippocampal electrode insertion". The Journal of Neuroscience. 26 (9): 2413–2418. doi:10.1523/JNEUROSCI.3680-05.2006. PMC 6793656. PMID 16510718.
- Mazroui R, Di Marco S, Kaufman RJ, Gallouzi IE (July 2007). "Inhibition of the ubiquitin-proteasome system induces stress granule formation". Molecular Biology of the Cell. 18 (7): 2603–2618. doi:10.1091/mbc.E06-12-1079. PMC 1924830. PMID 17475769.
- Frydryskova K, Masek T, Borcin K, Mrvova S, Venturi V, Pospisek M (August 2016). "Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules". BMC Molecular Biology. 17 (1): 21. doi:10.1186/s12867-016-0072-x. PMC 5006505. PMID 27578149.
- Battle DJ, Kasim M, Wang J, Dreyfuss G (September 2007). "SMN-independent subunits of the SMN complex. Identification of a small nuclear ribonucleoprotein assembly intermediate". The Journal of Biological Chemistry. 282 (38): 27953–27959. doi:10.1074/jbc.M702317200. PMID 17640873.
- Kim WJ, Back SH, Kim V, Ryu I, Jang SK (March 2005). "Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions". Molecular and Cellular Biology. 25 (6): 2450–2462. doi:10.1128/MCB.25.6.2450-2462.2005. PMC 1061607. PMID 15743837.
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M (November 2008). "Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways". Nature Cell Biology. 10 (11): 1324–1332. doi:10.1038/ncb1791. PMID 18836437. S2CID 21242075.
- Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA (March 2000). "HuR binding to cytoplasmic mRNA is perturbed by heat shock". Proceedings of the National Academy of Sciences of the United States of America. 97 (7): 3073–3078. Bibcode:2000PNAS...97.3073G. doi:10.1073/pnas.97.7.3073. PMC 16194. PMID 10737787.
- Thomas MG, Martinez Tosar LJ, Loschi M, Pasquini JM, Correale J, Kindler S, Boccaccio GL (January 2005). "Staufen recruitment into stress granules does not affect early mRNA transport in oligodendrocytes". Molecular Biology of the Cell. 16 (1): 405–420. doi:10.1091/mbc.E04-06-0516. PMC 539183. PMID 15525674.
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, et al. (November 2009). "TDP-43 is recruited to stress granules in conditions of oxidative insult". Journal of Neurochemistry. 111 (4): 1051–1061. doi:10.1111/j.1471-4159.2009.06383.x. PMID 19765185. S2CID 8630114.
- Meyerowitz J, Parker SJ, Vella LJ, Ng DC, Price KA, Liddell JR, et al. (August 2011). "C-Jun N-terminal kinase controls TDP-43 accumulation in stress granules induced by oxidative stress". Molecular Neurodegeneration. 6: 57. doi:10.1186/1750-1326-6-57. PMC 3162576. PMID 21819629.
- Burry RW, Smith CL (October 2006). "HuD distribution changes in response to heat shock but not neurotrophic stimulation". The Journal of Histochemistry and Cytochemistry. 54 (10): 1129–1138. doi:10.1369/jhc.6A6979.2006. PMC 3957809. PMID 16801526.
- Nawaz MS, Vik ES, Berges N, Fladeby C, Bjørås M, Dalhus B, Alseth I (October 2016). "Regulation of Human Endonuclease V Activity and Relocalization to Cytoplasmic Stress Granules". The Journal of Biological Chemistry. 291 (41): 21786–21801. doi:10.1074/jbc.M116.730911. PMC 5076846. PMID 27573237.
- Andersson MK, Ståhlberg A, Arvidsson Y, Olofsson A, Semb H, Stenman G, et al. (July 2008). "The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response". BMC Cell Biology. 9: 37. doi:10.1186/1471-2121-9-37. PMC 2478660. PMID 18620564.
- Neumann M, Bentmann E, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, et al. (September 2011). "FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations". Brain. 134 (Pt 9): 2595–2609. doi:10.1093/brain/awr201. PMC 3170539. PMID 21856723.
- Ozeki K, Sugiyama M, Akter KA, Nishiwaki K, Asano-Inami E, Senga T (January 2019). "FAM98A is localized to stress granules and associates with multiple stress granule-localized proteins". Molecular and Cellular Biochemistry. 451 (1–2): 107–115. doi:10.1007/s11010-018-3397-6. PMID 29992460. S2CID 49667042.
- Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW (November 2002). "Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression". Human Molecular Genetics. 11 (24): 3007–3017. doi:10.1093/hmg/11.24.3007. PMID 12417522.
- Dolzhanskaya N, Merz G, Denman RB (September 2006). "Oxidative stress reveals heterogeneity of FMRP granules in PC12 cell neurites". Brain Research. 1112 (1): 56–64. doi:10.1016/j.brainres.2006.07.026. PMID 16919243. S2CID 41514888.
- Blechingberg J, Luo Y, Bolund L, Damgaard CK, Nielsen AL (2012). "Gene expression responses to FUS, EWS, and TAF15 reduction and stress granule sequestration analyses identifies FET-protein non-redundant functions". PLOS ONE. 7 (9): e46251. Bibcode:2012PLoSO...746251B. doi:10.1371/journal.pone.0046251. PMC 3457980. PMID 23049996.
- Sama RR, Ward CL, Kaushansky LJ, Lemay N, Ishigaki S, Urano F, Bosco DA (November 2013). "FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress". Journal of Cellular Physiology. 228 (11): 2222–2231. doi:10.1002/jcp.24395. PMC 4000275. PMID 23625794.
- Di Salvio M, Piccinni V, Gerbino V, Mantoni F, Camerini S, Lenzi J, et al. (October 2015). "Pur-alpha functionally interacts with FUS carrying ALS-associated mutations". Cell Death & Disease. 6 (10): e1943. doi:10.1038/cddis.2015.295. PMC 4632316. PMID 26492376.
- Lenzi J, De Santis R, de Turris V, Morlando M, Laneve P, Calvo A, et al. (July 2015). "ALS mutant FUS proteins are recruited into stress granules in induced pluripotent stem cell-derived motoneurons". Disease Models & Mechanisms. 8 (7): 755–766. doi:10.1242/dmm.020099. PMC 4486861. PMID 26035390.
- Daigle JG, Krishnamurthy K, Ramesh N, Casci I, Monaghan J, McAvoy K, et al. (April 2016). "Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity". Acta Neuropathologica. 131 (4): 605–620. doi:10.1007/s00401-015-1530-0. PMC 4791193. PMID 26728149.
- Lo Bello M, Di Fini F, Notaro A, Spataro R, Conforti FL, La Bella V (2017-10-17). "ALS-Related Mutant FUS Protein Is Mislocalized to Cytoplasm and Is Recruited into Stress Granules of Fibroblasts from Asymptomatic FUS P525L Mutation Carriers". Neuro-Degenerative Diseases. 17 (6): 292–303. doi:10.1159/000480085. PMID 29035885. S2CID 40561105.
- Marrone L, Poser I, Casci I, Japtok J, Reinhardt P, Janosch A, et al. (February 2018). "Isogenic FUS-eGFP iPSC Reporter Lines Enable Quantification of FUS Stress Granule Pathology that Is Rescued by Drugs Inducing Autophagy". Stem Cell Reports. 10 (2): 375–389. doi:10.1016/j.stemcr.2017.12.018. PMC 5857889. PMID 29358088.
- Hofmann I, Casella M, Schnölzer M, Schlechter T, Spring H, Franke WW (March 2006). "Identification of the junctional plaque protein plakophilin 3 in cytoplasmic particles containing RNA-binding proteins and the recruitment of plakophilins 1 and 3 to stress granules". Molecular Biology of the Cell. 17 (3): 1388–1398. doi:10.1091/mbc.E05-08-0708. PMC 1382326. PMID 16407409.
- Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J (March 2003). "The RasGAP-associated endoribonuclease G3BP assembles stress granules". The Journal of Cell Biology. 160 (6): 823–831. doi:10.1083/jcb.200212128. PMC 2173781. PMID 12642610.
- Hua Y, Zhou J (January 2004). "Rpp20 interacts with SMN and is re-distributed into SMN granules in response to stress". Biochemical and Biophysical Research Communications. 314 (1): 268–276. doi:10.1016/j.bbrc.2003.12.084. PMID 14715275.
- Kwon S, Zhang Y, Matthias P (December 2007). "The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response". Genes & Development. 21 (24): 3381–3394. doi:10.1101/gad.461107. PMC 2113037. PMID 18079183.
- Tsai WC, Reineke LC, Jain A, Jung SY, Lloyd RE (November 2017). "Histone arginine demethylase JMJD6 is linked to stress granule assembly through demethylation of the stress granule-nucleating protein G3BP1". The Journal of Biological Chemistry. 292 (46): 18886–18896. doi:10.1074/jbc.M117.800706. PMC 5704473. PMID 28972166.
- Kobayashi T, Winslow S, Sunesson L, Hellman U, Larsson C (2012). "PKCα binds G3BP2 and regulates stress granule formation following cellular stress". PLOS ONE. 7 (4): e35820. Bibcode:2012PLoSO...735820K. doi:10.1371/journal.pone.0035820. PMC 3335008. PMID 22536444.
- Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M (February 2013). "Both G3BP1 and G3BP2 contribute to stress granule formation". Genes to Cells. 18 (2): 135–146. doi:10.1111/gtc.12023. PMID 23279204. S2CID 11859927.
- Folkmann AW, Wente SR (April 2015). "Cytoplasmic hGle1A regulates stress granules by modulation of translation". Molecular Biology of the Cell. 26 (8): 1476–1490. doi:10.1091/mbc.E14-11-1523. PMC 4395128. PMID 25694449.
- Zhang K, Daigle JG, Cunningham KM, Coyne AN, Ruan K, Grima JC, et al. (May 2018). "Stress Granule Assembly Disrupts Nucleocytoplasmic Transport". Cell. 173 (4): 958–971.e17. doi:10.1016/j.cell.2018.03.025. PMC 6083872. PMID 29628143.
- Tsai NP, Ho PC, Wei LN (March 2008). "Regulation of stress granule dynamics by Grb7 and FAK signalling pathway". The EMBO Journal. 27 (5): 715–726. doi:10.1038/emboj.2008.19. PMC 2265756. PMID 18273060.
- Krisenko MO, Higgins RL, Ghosh S, Zhou Q, Trybula JS, Wang WH, Geahlen RL (November 2015). "Syk Is Recruited to Stress Granules and Promotes Their Clearance through Autophagy". The Journal of Biological Chemistry. 290 (46): 27803–27815. doi:10.1074/jbc.M115.642900. PMC 4646026. PMID 26429917.
- Grousl T, Ivanov P, Malcova I, Pompach P, Frydlova I, Slaba R, et al. (2013). "Heat shock-induced accumulation of translation elongation and termination factors precedes assembly of stress granules in S. cerevisiae". PLOS ONE. 8 (2): e57083. Bibcode:2013PLoSO...857083G. doi:10.1371/journal.pone.0057083. PMC 3581570. PMID 23451152.
- Gonçalves K, Bressan GC, Saito A, Morello LG, Zanchin NI, Kobarg J (August 2011). "Evidence for the association of the human regulatory protein Ki-1/57 with the translational machinery". FEBS Letters. 585 (16): 2556–2560. doi:10.1016/j.febslet.2011.07.010. PMID 21771594.
- Guil S, Long JC, Cáceres JF (August 2006). "hnRNP A1 relocalization to the stress granules reflects a role in the stress response". Molecular and Cellular Biology. 26 (15): 5744–5758. doi:10.1128/MCB.00224-06. PMC 1592774. PMID 16847328.
- Dewey CM, Cenik B, Sephton CF, Dries DR, Mayer P, Good SK, et al. (March 2011). "TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor". Molecular and Cellular Biology. 31 (5): 1098–1108. doi:10.1128/MCB.01279-10. PMC 3067820. PMID 21173160.
- Papadopoulou C, Ganou V, Patrinou-Georgoula M, Guialis A (January 2013). "HuR-hnRNP interactions and the effect of cellular stress". Molecular and Cellular Biochemistry. 372 (1–2): 137–147. doi:10.1007/s11010-012-1454-0. PMID 22983828. S2CID 16261648.
- Naruse H, Ishiura H, Mitsui J, Date H, Takahashi Y, Matsukawa T, et al. (January 2018). "Molecular epidemiological study of familial amyotrophic lateral sclerosis in Japanese population by whole-exome sequencing and identification of novel HNRNPA1 mutation". Neurobiology of Aging. 61: 255.e9–255.e16. doi:10.1016/j.neurobiolaging.2017.08.030. PMID 29033165. S2CID 38838445.
- McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, et al. (April 2011). "TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1". Human Molecular Genetics. 20 (7): 1400–1410. doi:10.1093/hmg/ddr021. PMID 21257637.
- Fukuda T, Naiki T, Saito M, Irie K (February 2009). "hnRNP K interacts with RNA binding motif protein 42 and functions in the maintenance of cellular ATP level during stress conditions". Genes to Cells. 14 (2): 113–128. doi:10.1111/j.1365-2443.2008.01256.x. PMID 19170760. S2CID 205293176.
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P (December 1999). "RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules". The Journal of Cell Biology. 147 (7): 1431–1442. doi:10.1083/jcb.147.7.1431. PMC 2174242. PMID 10613902.
- Ganassi M, Mateju D, Bigi I, Mediani L, Poser I, Lee HO, et al. (September 2016). "A Surveillance Function of the HSPB8-BAG3-HSP70 Chaperone Complex Ensures Stress Granule Integrity and Dynamism". Molecular Cell. 63 (5): 796–810. doi:10.1016/j.molcel.2016.07.021. PMID 27570075.
- Mahboubi H, Moujaber O, Kodiha M, Stochaj U (March 2020). "The Co-Chaperone HspBP1 Is a Novel Component of Stress Granules that Regulates Their Formation". Cells. 9 (4): 825. doi:10.3390/cells9040825. PMC 7226807. PMID 32235396.
- Wen X, Huang X, Mok BW, Chen Y, Zheng M, Lau SY, et al. (April 2014). "NF90 exerts antiviral activity through regulation of PKR phosphorylation and stress granules in infected cells". Journal of Immunology. 192 (8): 3753–3764. doi:10.4049/jimmunol.1302813. PMID 24623135.
- Brehm MA, Schenk TM, Zhou X, Fanick W, Lin H, Windhorst S, et al. (December 2007). "Intracellular localization of human Ins(1,3,4,5,6)P5 2-kinase". The Biochemical Journal. 408 (3): 335–345. doi:10.1042/BJ20070382. PMC 2267366. PMID 17705785.
- Piotrowska J, Hansen SJ, Park N, Jamka K, Sarnow P, Gustin KE (April 2010). "Stable formation of compositionally unique stress granules in virus-infected cells". Journal of Virology. 84 (7): 3654–3665. doi:10.1128/JVI.01320-09. PMC 2838110. PMID 20106928.
- Henao-Mejia J, He JJ (November 2009). "Sam68 relocalization into stress granules in response to oxidative stress through complexing with TIA-1". Experimental Cell Research. 315 (19): 3381–3395. doi:10.1016/j.yexcr.2009.07.011. PMC 2783656. PMID 19615357.
- Zhang H, Chen N, Li P, Pan Z, Ding Y, Zou D, et al. (July 2016). "The nuclear protein Sam68 is recruited to the cytoplasmic stress granules during enterovirus 71 infection". Microbial Pathogenesis. 96: 58–66. doi:10.1016/j.micpath.2016.04.001. PMID 27057671.
- Rothé F, Gueydan C, Bellefroid E, Huez G, Kruys V (April 2006). "Identification of FUSE-binding proteins as interacting partners of TIA proteins". Biochemical and Biophysical Research Communications. 343 (1): 57–68. doi:10.1016/j.bbrc.2006.02.112. PMID 16527256.
- Mahboubi H, Seganathy E, Kong D, Stochaj U (2013). "Identification of Novel Stress Granule Components That Are Involved in Nuclear Transport". PLOS ONE. 8 (6): e68356. Bibcode:2013PLoSO...868356M. doi:10.1371/journal.pone.0068356. PMC 3694919. PMID 23826389.
- Fujimura K, Suzuki T, Yasuda Y, Murata M, Katahira J, Yoneda Y (July 2010). "Identification of importin alpha1 as a novel constituent of RNA stress granules". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1803 (7): 865–871. doi:10.1016/j.bbamcr.2010.03.020. PMID 20362631.
- Yang R, Gaidamakov SA, Xie J, Lee J, Martino L, Kozlov G, et al. (February 2011). "La-related protein 4 binds poly(A), interacts with the poly(A)-binding protein MLLE domain via a variant PAM2w motif, and can promote mRNA stability". Molecular and Cellular Biology. 31 (3): 542–556. doi:10.1128/MCB.01162-10. PMC 3028612. PMID 21098120.
- Balzer E, Moss EG (January 2007). "Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules". RNA Biology. 4 (1): 16–25. doi:10.4161/rna.4.1.4364. PMID 17617744.
- Ingelfinger D, Arndt-Jovin DJ, Lührmann R, Achsel T (December 2002). "The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci". RNA. 8 (12): 1489–1501. doi:10.1017/S1355838202021726. PMC 1370355. PMID 12515382.
- Yang WH, Yu JH, Gulick T, Bloch KD, Bloch DB (April 2006). "RNA-associated protein 55 (RAP55) localizes to mRNA processing bodies and stress granules". RNA. 12 (4): 547–554. doi:10.1261/rna.2302706. PMC 1421083. PMID 16484376.
- Kawahara H, Imai T, Imataka H, Tsujimoto M, Matsumoto K, Okano H (May 2008). "Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP". The Journal of Cell Biology. 181 (4): 639–653. doi:10.1083/jcb.200708004. PMC 2386104. PMID 18490513.
- Yuan L, Xiao Y, Zhou Q, Yuan D, Wu B, Chen G, Zhou J (January 2014). "Proteomic analysis reveals that MAEL, a component of nuage, interacts with stress granule proteins in cancer cells". Oncology Reports. 31 (1): 342–350. doi:10.3892/or.2013.2836. PMID 24189637.
- Seguin SJ, Morelli FF, Vinet J, Amore D, De Biasi S, Poletti A, et al. (December 2014). "Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly". Cell Death and Differentiation. 21 (12): 1838–1851. doi:10.1038/cdd.2014.103. PMC 4227144. PMID 25034784.
- Ryu HH, Jun MH, Min KJ, Jang DJ, Lee YS, Kim HK, Lee JA (December 2014). "Autophagy regulates amyotrophic lateral sclerosis-linked fused in sarcoma-positive stress granules in neurons". Neurobiology of Aging. 35 (12): 2822–2831. doi:10.1016/j.neurobiolaging.2014.07.026. PMID 25216585. S2CID 36917292.
- Wasserman T, Katsenelson K, Daniliuc S, Hasin T, Choder M, Aronheim A (January 2010). "A novel c-Jun N-terminal kinase (JNK)-binding protein WDR62 is recruited to stress granules and mediates a nonclassical JNK activation". Molecular Biology of the Cell. 21 (1): 117–130. doi:10.1091/mbc.E09-06-0512. PMC 2801705. PMID 19910486.
- Courchet J, Buchet-Poyau K, Potemski A, Brès A, Jariel-Encontre I, Billaud M (November 2008). "Interaction with 14-3-3 adaptors regulates the sorting of hMex-3B RNA-binding protein to distinct classes of RNA granules". The Journal of Biological Chemistry. 283 (46): 32131–32142. doi:10.1074/jbc.M802927200. PMID 18779327.
- Kuniyoshi K, Takeuchi O, Pandey S, Satoh T, Iwasaki H, Akira S, Kawai T (April 2014). "Pivotal role of RNA-binding E3 ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity". Proceedings of the National Academy of Sciences of the United States of America. 111 (15): 5646–5651. Bibcode:2014PNAS..111.5646K. doi:10.1073/pnas.1401674111. PMC 3992669. PMID 24706898.
- ErLin S, WenJie W, LiNing W, BingXin L, MingDe L, Yan S, RuiFa H (May 2015). "Musashi-1 maintains blood-testis barrier structure during spermatogenesis and regulates stress granule formation upon heat stress". Molecular Biology of the Cell. 26 (10): 1947–1956. doi:10.1091/mbc.E14-11-1497. PMC 4436837. PMID 25717188.
- MacNair L, Xiao S, Miletic D, Ghani M, Julien JP, Keith J, et al. (January 2016). "MTHFSD and DDX58 are novel RNA-binding proteins abnormally regulated in amyotrophic lateral sclerosis". Brain. 139 (Pt 1): 86–100. doi:10.1093/brain/awv308. PMID 26525917.
- Sfakianos AP, Mellor LE, Pang YF, Kritsiligkou P, Needs H, Abou-Hamdan H, et al. (November 2018). "The mTOR-S6 kinase pathway promotes stress granule assembly". Cell Death and Differentiation. 25 (10): 1766–1780. doi:10.1038/s41418-018-0076-9. PMC 6004310. PMID 29523872.
- Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW (March 2007). "An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response". Molecular Cell. 25 (5): 765–778. doi:10.1016/j.molcel.2007.01.025. PMC 1864954. PMID 17349961.
- Furukawa MT, Sakamoto H, Inoue K (April 2015). "Interaction and colocalization of HERMES/RBPMS with NonO, PSF, and G3BP1 in neuronal cytoplasmic RNP granules in mouse retinal line cells". Genes to Cells. 20 (4): 257–266. doi:10.1111/gtc.12224. PMID 25651939. S2CID 22403884.
- Kang JS, Hwang YS, Kim LK, Lee S, Lee WB, Kim-Ha J, Kim YJ (March 2018). "OASL1 Traps Viral RNAs in Stress Granules to Promote Antiviral Responses". Molecules and Cells. 41 (3): 214–223. doi:10.14348/molcells.2018.2293. PMC 5881095. PMID 29463066.
- Wehner KA, Schütz S, Sarnow P (April 2010). "OGFOD1, a novel modulator of eukaryotic translation initiation factor 2alpha phosphorylation and the cellular response to stress". Molecular and Cellular Biology. 30 (8): 2006–2016. doi:10.1128/MCB.01350-09. PMC 2849474. PMID 20154146.
- Bravard A, Campalans A, Vacher M, Gouget B, Levalois C, Chevillard S, Radicella JP (March 2010). "Inactivation by oxidation and recruitment into stress granules of hOGG1 but not APE1 in human cells exposed to sub-lethal concentrations of cadmium". Mutation Research. 685 (1–2): 61–69. doi:10.1016/j.mrfmmm.2009.09.013. PMID 19800894.
- Das R, Schwintzer L, Vinopal S, Aguado Roca E, Sylvester M, Oprisoreanu AM, et al. (June 2019). "New roles for the de-ubiquitylating enzyme OTUD4 in an RNA-protein network and RNA granules". Journal of Cell Science. 132 (12): jcs229252. doi:10.1242/jcs.229252. PMC 6602300. PMID 31138677.
- Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P (May 2011). "Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm". Molecular Cell. 42 (4): 489–499. doi:10.1016/j.molcel.2011.04.015. PMC 3898460. PMID 21596313.
- Repici M, Hassanjani M, Maddison DC, Garção P, Cimini S, Patel B, et al. (January 2019). "The Parkinson's Disease-Linked Protein DJ-1 Associates with Cytoplasmic mRNP Granules During Stress and Neurodegeneration". Molecular Neurobiology. 56 (1): 61–77. doi:10.1007/s12035-018-1084-y. PMC 6334738. PMID 29675578.
- Catara G, Grimaldi G, Schembri L, Spano D, Turacchio G, Lo Monte M, et al. (October 2017). "PARP1-produced poly-ADP-ribose causes the PARP12 translocation to stress granules and impairment of Golgi complex functions". Scientific Reports. 7 (1): 14035. Bibcode:2017NatSR...714035C. doi:10.1038/s41598-017-14156-8. PMC 5656619. PMID 29070863.
- Bai Y, Dong Z, Shang Q, Zhao H, Wang L, Guo C, et al. (2016). "Pdcd4 Is Involved in the Formation of Stress Granule in Response to Oxidized Low-Density Lipoprotein or High-Fat Diet". PLOS ONE. 11 (7): e0159568. Bibcode:2016PLoSO..1159568B. doi:10.1371/journal.pone.0159568. PMC 4959751. PMID 27454120.
- Kunde SA, Musante L, Grimme A, Fischer U, Müller E, Wanker EE, Kalscheuer VM (December 2011). "The X-chromosome-linked intellectual disability protein PQBP1 is a component of neuronal RNA granules and regulates the appearance of stress granules". Human Molecular Genetics. 20 (24): 4916–4931. doi:10.1093/hmg/ddr430. PMID 21933836.
- Turakhiya A, Meyer SR, Marincola G, Böhm S, Vanselow JT, Schlosser A, et al. (June 2018). "ZFAND1 Recruits p97 and the 26S Proteasome to Promote the Clearance of Arsenite-Induced Stress Granules". Molecular Cell. 70 (5): 906–919.e7. doi:10.1016/j.molcel.2018.04.021. PMID 29804830.
- Yang F, Peng Y, Murray EL, Otsuka Y, Kedersha N, Schoenberg DR (December 2006). "Polysome-bound endonuclease PMR1 is targeted to stress granules via stress-specific binding to TIA-1". Molecular and Cellular Biology. 26 (23): 8803–8813. doi:10.1128/MCB.00090-06. PMC 1636822. PMID 16982678.
- Takahashi M, Higuchi M, Matsuki H, Yoshita M, Ohsawa T, Oie M, Fujii M (February 2013). "Stress granules inhibit apoptosis by reducing reactive oxygen species production". Molecular and Cellular Biology. 33 (4): 815–829. doi:10.1128/MCB.00763-12. PMC 3571346. PMID 23230274.
- Park C, Choi S, Kim YE, Lee S, Park SH, Adelstein RS, et al. (September 2017). "Stress Granules Contain Rbfox2 with Cell Cycle-related mRNAs". Scientific Reports. 7 (1): 11211. Bibcode:2017NatSR...711211P. doi:10.1038/s41598-017-11651-w. PMC 5593835. PMID 28894257.
- Kucherenko MM, Shcherbata HR (January 2018). "Stress-dependent miR-980 regulation of Rbfox1/A2bp1 promotes ribonucleoprotein granule formation and cell survival". Nature Communications. 9 (1): 312. Bibcode:2018NatCo...9..312K. doi:10.1038/s41467-017-02757-w. PMC 5778076. PMID 29358748.
- Lin JC, Hsu M, Tarn WY (February 2007). "Cell stress modulates the function of splicing regulatory protein RBM4 in translation control". Proceedings of the National Academy of Sciences of the United States of America. 104 (7): 2235–2240. Bibcode:2007PNAS..104.2235L. doi:10.1073/pnas.0611015104. PMC 1893002. PMID 17284590.
- Bakkar N, Kousari A, Kovalik T, Li Y, Bowser R (July 2015). "RBM45 Modulates the Antioxidant Response in Amyotrophic Lateral Sclerosis through Interactions with KEAP1". Molecular and Cellular Biology. 35 (14): 2385–2399. doi:10.1128/MCB.00087-15. PMC 4475920. PMID 25939382.
- Li Y, Collins M, Geiser R, Bakkar N, Riascos D, Bowser R (September 2015). "RBM45 homo-oligomerization mediates association with ALS-linked proteins and stress granules". Scientific Reports. 5: 14262. Bibcode:2015NatSR...514262L. doi:10.1038/srep14262. PMC 4585734. PMID 26391765.
- Farazi TA, Leonhardt CS, Mukherjee N, Mihailovic A, Li S, Max KE, et al. (July 2014). "Identification of the RNA recognition element of the RBPMS family of RNA-binding proteins and their transcriptome-wide mRNA targets". RNA. 20 (7): 1090–1102. doi:10.1261/rna.045005.114. PMC 4114688. PMID 24860013.
- Athanasopoulos V, Barker A, Yu D, Tan AH, Srivastava M, Contreras N, et al. (May 2010). "The ROQUIN family of proteins localizes to stress granules via the ROQ domain and binds target mRNAs". The FEBS Journal. 277 (9): 2109–2127. doi:10.1111/j.1742-4658.2010.07628.x. PMID 20412057. S2CID 13387108.
- Eisinger-Mathason TS, Andrade J, Groehler AL, Clark DE, Muratore-Schroeder TL, Pasic L, et al. (September 2008). "Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival". Molecular Cell. 31 (5): 722–736. doi:10.1016/j.molcel.2008.06.025. PMC 2654589. PMID 18775331.
- Baez MV, Boccaccio GL (December 2005). "Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules". The Journal of Biological Chemistry. 280 (52): 43131–43140. doi:10.1074/jbc.M508374200. PMID 16221671.
- Lee YJ, Wei HM, Chen LY, Li C (January 2014). "Localization of SERBP1 in stress granules and nucleoli". The FEBS Journal. 281 (1): 352–364. doi:10.1111/febs.12606. PMID 24205981. S2CID 20464730.
- Omer A, Patel D, Lian XJ, Sadek J, Di Marco S, Pause A, et al. (May 2018). "Stress granules counteract senescence by sequestration of PAI-1". EMBO Reports. 19 (5): e44722. doi:10.15252/embr.201744722. PMC 5934773. PMID 29592859.
- Jedrusik-Bode M, Studencka M, Smolka C, Baumann T, Schmidt H, Kampf J, et al. (November 2013). "The sirtuin SIRT6 regulates stress granule formation in C. elegans and mammals". Journal of Cell Science. 126 (Pt 22): 5166–5177. doi:10.1242/jcs.130708. PMID 24013546.
- Brown JA, Roberts TL, Richards R, Woods R, Birrell G, Lim YC, et al. (November 2011). "A novel role for hSMG-1 in stress granule formation". Molecular and Cellular Biology. 31 (22): 4417–4429. doi:10.1128/MCB.05987-11. PMC 3209244. PMID 21911475.
- Hua Y, Zhou J (August 2004). "Survival motor neuron protein facilitates assembly of stress granules". FEBS Letters. 572 (1–3): 69–74. doi:10.1016/j.febslet.2004.07.010. PMID 15304326. S2CID 27599172.
- Zou T, Yang X, Pan D, Huang J, Sahin M, Zhou J (May 2011). "SMN deficiency reduces cellular ability to form stress granules, sensitizing cells to stress". Cellular and Molecular Neurobiology. 31 (4): 541–550. doi:10.1007/s10571-011-9647-8. PMID 21234798. S2CID 8763933.
- Gao X, Fu X, Song J, Zhang Y, Cui X, Su C, et al. (March 2015). "Poly(A)(+) mRNA-binding protein Tudor-SN regulates stress granules aggregation dynamics". The FEBS Journal. 282 (5): 874–890. doi:10.1111/febs.13186. PMID 25559396. S2CID 27524910.
- Chang YW, Huang YS (2014). "Arsenite-activated JNK signaling enhances CPEB4-Vinexin interaction to facilitate stress granule assembly and cell survival". PLOS ONE. 9 (9): e107961. Bibcode:2014PLoSO...9j7961C. doi:10.1371/journal.pone.0107961. PMC 4169592. PMID 25237887.
- Zhu CH, Kim J, Shay JW, Wright WE (2008). "SGNP: an essential Stress Granule/Nucleolar Protein potentially involved in 5.8s rRNA processing/transport". PLOS ONE. 3 (11): e3716. Bibcode:2008PLoSO...3.3716Z. doi:10.1371/journal.pone.0003716. PMC 2579992. PMID 19005571.
- Berger A, Ivanova E, Gareau C, Scherrer A, Mazroui R, Strub K (2014). "Direct binding of the Alu binding protein dimer SRP9/14 to 40S ribosomal subunits promotes stress granule formation and is regulated by Alu RNA". Nucleic Acids Research. 42 (17): 11203–11217. doi:10.1093/nar/gku822. PMC 4176187. PMID 25200073.
- Delestienne N, Wauquier C, Soin R, Dierick JF, Gueydan C, Kruys V (June 2010). "The splicing factor ASF/SF2 is associated with TIA-1-related/TIA-1-containing ribonucleoproteic complexes and contributes to post-transcriptional repression of gene expression". The FEBS Journal. 277 (11): 2496–2514. doi:10.1111/j.1742-4658.2010.07664.x. PMID 20477871. S2CID 24332251.
- Fitzgerald KD, Semler BL (September 2013). "Poliovirus infection induces the co-localization of cellular protein SRp20 with TIA-1, a cytoplasmic stress granule protein". Virus Research. 176 (1–2): 223–231. doi:10.1016/j.virusres.2013.06.012. PMC 3742715. PMID 23830997.
- Kano S, Nishida K, Kurebe H, Nishiyama C, Kita K, Akaike Y, et al. (February 2014). "Oxidative stress-inducible truncated serine/arginine-rich splicing factor 3 regulates interleukin-8 production in human colon cancer cells". American Journal of Physiology. Cell Physiology. 306 (3): C250–C262. doi:10.1152/ajpcell.00091.2013. PMID 24284797. S2CID 17352565.
- Jayabalan AK, Sanchez A, Park RY, Yoon SP, Kang GY, Baek JH, et al. (July 2016). "NEDDylation promotes stress granule assembly". Nature Communications. 7: 12125. Bibcode:2016NatCo...712125J. doi:10.1038/ncomms12125. PMC 4935812. PMID 27381497.
- Kukharsky MS, Quintiero A, Matsumoto T, Matsukawa K, An H, Hashimoto T, et al. (April 2015). "Calcium-responsive transactivator (CREST) protein shares a set of structural and functional traits with other proteins associated with amyotrophic lateral sclerosis". Molecular Neurodegeneration. 10: 20. doi:10.1186/s13024-015-0014-y. PMC 4428507. PMID 25888396.
- Thomas MG, Martinez Tosar LJ, Desbats MA, Leishman CC, Boccaccio GL (February 2009). "Mammalian Staufen 1 is recruited to stress granules and impairs their assembly". Journal of Cell Science. 122 (Pt 4): 563–573. doi:10.1242/jcs.038208. PMC 2714435. PMID 19193871.
- Quaresma AJ, Bressan GC, Gava LM, Lanza DC, Ramos CH, Kobarg J (April 2009). "Human hnRNP Q re-localizes to cytoplasmic granules upon PMA, thapsigargin, arsenite and heat-shock treatments". Experimental Cell Research. 315 (6): 968–980. doi:10.1016/j.yexcr.2009.01.012. PMID 19331829.
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Vanderwyde T, Citro A, et al. (October 2010). "Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue". PLOS ONE. 5 (10): e13250. Bibcode:2010PLoSO...513250L. doi:10.1371/journal.pone.0013250. PMC 2952586. PMID 20948999.
- Freibaum BD, Chitta RK, High AA, Taylor JP (February 2010). "Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery". Journal of Proteome Research. 9 (2): 1104–1120. doi:10.1021/pr901076y. PMC 2897173. PMID 20020773.
- Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, et al. (August 2017). "TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics". Neuron (Submitted manuscript). 95 (4): 808–816.e9. doi:10.1016/j.neuron.2017.07.025. PMC 5576574. PMID 28817800.
- Khalfallah Y, Kuta R, Grasmuck C, Prat A, Durham HD, Vande Velde C (May 2018). "TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types". Scientific Reports. 8 (1): 7551. Bibcode:2018NatSR...8.7551K. doi:10.1038/s41598-018-25767-0. PMC 5953947. PMID 29765078.
- Linder B, Plöttner O, Kroiss M, Hartmann E, Laggerbauer B, Meister G, et al. (October 2008). "Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP". Human Molecular Genetics. 17 (20): 3236–3246. doi:10.1093/hmg/ddn219. PMID 18664458.
- Stoll G, Pietiläinen OP, Linder B, Suvisaari J, Brosi C, Hennah W, et al. (September 2013). "Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders". Nature Neuroscience. 16 (9): 1228–1237. doi:10.1038/nn.3484. PMC 3986889. PMID 23912948.
- Narayanan N, Wang Z, Li L, Yang Y (2017). "Arginine methylation of USP9X promotes its interaction with TDRD3 and its anti-apoptotic activities in breast cancer cells". Cell Discovery. 3: 16048. doi:10.1038/celldisc.2016.48. PMC 5206711. PMID 28101374.
- Iannilli F, Zalfa F, Gartner A, Bagni C, Dotti CG (2013). "Cytoplasmic TERT Associates to RNA Granules in Fully Mature Neurons: Role in the Translational Control of the Cell Cycle Inhibitor p15INK4B". PLOS ONE. 8 (6): e66602. Bibcode:2013PLoSO...866602I. doi:10.1371/journal.pone.0066602. PMC 3688952. PMID 23825548.
- Lee Y, Jonson PH, Sarparanta J, Palmio J, Sarkar M, Vihola A, et al. (March 2018). "TIA1 variant drives myodegeneration in multisystem proteinopathy with SQSTM1 mutations". The Journal of Clinical Investigation. 128 (3): 1164–1177. doi:10.1172/JCI97103. PMC 5824866. PMID 29457785.
- Chang WL, Tarn WY (October 2009). "A role for transportin in deposition of TTP to cytoplasmic RNA granules and mRNA decay". Nucleic Acids Research. 37 (19): 6600–6612. doi:10.1093/nar/gkp717. PMC 2770677. PMID 19729507.
- Guo L, Kim HJ, Wang H, Monaghan J, Freyermuth F, Sung JC, et al. (April 2018). "Nuclear-Import Receptors Reverse Aberrant Phase Transitions of RNA-Binding Proteins with Prion-like Domains". Cell. 173 (3): 677–692.e20. doi:10.1016/j.cell.2018.03.002. PMC 5911940. PMID 29677512.
- Huang L, Wang Z, Narayanan N, Yang Y (April 2018). "Arginine methylation of the C-terminus RGG motif promotes TOP3B topoisomerase activity and stress granule localization". Nucleic Acids Research. 46 (6): 3061–3074. doi:10.1093/nar/gky103. PMC 5888246. PMID 29471495.
- Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, Lyko F (August 2010). "RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage". Genes & Development. 24 (15): 1590–1595. doi:10.1101/gad.586710. PMC 2912555. PMID 20679393.
- Huang C, Chen Y, Dai H, Zhang H, Xie M, Zhang H, et al. (January 2020). "UBAP2L arginine methylation by PRMT1 modulates stress granule assembly". Cell Death and Differentiation. 27 (1): 227–241. doi:10.1038/s41418-019-0350-5. PMC 7205891. PMID 31114027.
- Cirillo L, Cieren A, Barbieri S, Khong A, Schwager F, Parker R, Gotta M (February 2020). "UBAP2L Forms Distinct Cores that Act in Nucleating Stress Granules Upstream of G3BP1". Current Biology. 30 (4): 698–707.e6. doi:10.1016/j.cub.2019.12.020. PMID 31956030. S2CID 210597276.
- Dao TP, Kolaitis RM, Kim HJ, O'Donovan K, Martyniak B, Colicino E, et al. (March 2018). "Ubiquitin Modulates Liquid-Liquid Phase Separation of UBQLN2 via Disruption of Multivalent Interactions". Molecular Cell. 69 (6): 965–978.e6. doi:10.1016/j.molcel.2018.02.004. PMC 6181577. PMID 29526694.
- Wang B, Maxwell BA, Joo JH, Gwon Y, Messing J, Mishra A, et al. (May 2019). "ULK1 and ULK2 Regulate Stress Granule Disassembly Through Phosphorylation and Activation of VCP/p97". Molecular Cell. 74 (4): 742–757.e8. doi:10.1016/j.molcel.2019.03.027. PMC 6859904. PMID 30979586.
- Xie X, Matsumoto S, Endo A, Fukushima T, Kawahara H, Saeki Y, Komada M (April 2018). "Deubiquitylases USP5 and USP13 are recruited to and regulate heat-induced stress granules through their deubiquitylating activities". Journal of Cell Science. 131 (8): jcs210856. doi:10.1242/jcs.210856. PMID 29567855.
- Buchan JR, Kolaitis RM, Taylor JP, Parker R (June 2013). "Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function". Cell. 153 (7): 1461–1474. doi:10.1016/j.cell.2013.05.037. PMC 3760148. PMID 23791177.
- Somasekharan SP, El-Naggar A, Leprivier G, Cheng H, Hajee S, Grunewald TG, et al. (March 2015). "YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1". The Journal of Cell Biology. 208 (7): 913–929. doi:10.1083/jcb.201411047. PMC 4384734. PMID 25800057.
- Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, et al. (July 2019). "m6A enhances the phase separation potential of mRNA". Nature. 571 (7765): 424–428. doi:10.1038/s41586-019-1374-1. PMC 6662915. PMID 31292544.
- Fu Y, Zhuang X (September 2020). "m6A-binding YTHDF proteins promote stress granule formation". Nature Chemical Biology. 16 (9): 955–963. doi:10.1038/s41589-020-0524-y. PMC 7442727. PMID 32451507.
- Stöhr N, Lederer M, Reinke C, Meyer S, Hatzfeld M, Singer RH, Hüttelmaier S (November 2006). "ZBP1 regulates mRNA stability during cellular stress". The Journal of Cell Biology. 175 (4): 527–534. doi:10.1083/jcb.200608071. PMC 2064588. PMID 17101699.
- Deigendesch N, Koch-Nolte F, Rothenburg S (2006). "ZBP1 subcellular localization and association with stress granules is controlled by its Z-DNA binding domains". Nucleic Acids Research. 34 (18): 5007–5020. doi:10.1093/nar/gkl575. PMC 1636418. PMID 16990255.
- Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P (March 2004). "MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay". The EMBO Journal. 23 (6): 1313–1324. doi:10.1038/sj.emboj.7600163. PMC 381421. PMID 15014438.
- Holmes B, Artinian N, Anderson L, Martin J, Masri J, Cloninger C, et al. (January 2012). "Protor-2 interacts with tristetraprolin to regulate mRNA stability during stress". Cellular Signalling. 24 (1): 309–315. doi:10.1016/j.cellsig.2011.09.015. PMC 3205320. PMID 21964062.
- Murata T, Morita N, Hikita K, Kiuchi K, Kiuchi K, Kaneda N (February 2005). "Recruitment of mRNA-destabilizing protein TIS11 to stress granules is mediated by its zinc finger domain". Experimental Cell Research. 303 (2): 287–299. doi:10.1016/j.yexcr.2004.09.031. PMID 15652343.
Further reading
- Anderson P, Kedersha N (March 2006). "RNA granules". The Journal of Cell Biology. 172 (6): 803–808. doi:10.1083/jcb.200512082. PMC 2063724. PMID 16520386.
- Kedersha N, Anderson P (November 2002). "Stress granules: sites of mRNA triage that regulate mRNA stability and translatability". Biochemical Society Transactions. 30 (Pt 6): 963–969. doi:10.1042/BST0300963. PMID 12440955. S2CID 2833183.
— molecular details of stress granule assembly & function - Sandqvist A, Sistonen L (January 2004). "Nuclear stress granules: the awakening of a sleeping beauty?". The Journal of Cell Biology. 164 (1): 15–17. doi:10.1083/jcb.200311102. PMC 2171964. PMID 14709538.
External links
Laboratories: