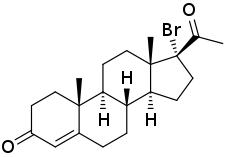
17α-Bromoprogesterone
17α-Bromoprogesterone (17α-BP) is a progestin which was first described in 1957 and was never marketed.[1][2][3][4] It is about twice as potent as progesterone in terms of progestogenic activity in animal bioassays.[1] 17α-BP is a parent compound of haloprogesterone (6α-fluoro-17α-bromoprogesterone) and 6α-methyl-17α-bromoprogesterone.[5]
 | |
| Clinical data | |
|---|---|
| Other names | 17α-BP; 17α-Bromopregn-4-ene-3,20-dione |
| Drug class | Progestogen; Progestin |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H29BrO2 |
| Molar mass | 393.365 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Engel CR, Jahnke H (November 1957). "Steroids and related products. X. 17 alpha-Bromoprogesterone, a new potent gestogen". Can J Biochem Physiol. 35 (11): 1047–55. doi:10.1139/o57-120. PMID 13479803.
- Seeley DH, Wang WY, Salhanick HA (November 1982). "Molecular interactions of progesterone analogues with rabbit uterine cytoplasmic receptor". J. Biol. Chem. 257 (22): 13359–66. doi:10.1016/S0021-9258(18)33456-2. PMID 7142152.
- Bohl M, Simon Z, Vlad A, Kaufmann G, Ponsold K (1987). "MTD calculations on quantitative structure-activity relationships of steroids binding to the progesterone receptor". Z. Naturforsch. C. 42 (7–8): 935–40. doi:10.1515/znc-1987-7-834. PMID 2961153. S2CID 22962904.
- Simon, Z.; Bohl, M. (1992). "Structure-activity Relations in Gestagenic Steroids by the MTD Method. The Case of Hard Molecules and Soft Receptors". Quantitative Structure-Activity Relationships. 11 (1): 23–28. doi:10.1002/qsar.19920110104. ISSN 0931-8771.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 179, 620. ISBN 978-1-4757-2085-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.