Trendione
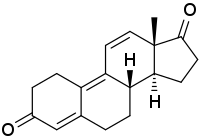
Trendione (developmental code name RU-2065; nickname Trenavar), also known as estra-4,9,11-triene-3,17-dione, is an androgen prohormone as well as metabolite of the anabolic steroid trenbolone.[1][2][3][4][5] Trendione is to trenbolone as androstenedione is to testosterone.[6] The compound is inactive itself, showing more than 100-fold lower affinity for the androgen and progesterone receptors than trenbolone.[7][8] It is a designer steroid and has been sold on the internet as a "nutritional supplement".[1] Trendione is listed in the United States Designer Anabolic Steroid Control Act of 2014.[1]
 | |
| Clinical data | |
|---|---|
| Other names | RU-2065; Trenavar; Triendione; Estra-4,9,11-triene-3,17-dione |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Liane BJ, Magee C (October 2016). "Guerilla Warfare on the Pancreas? A Case of Acute Pancreatitis From a Supplement Known to Contain Anabolic-Androgenic Steroids". Mil Med. 181 (10): e1395–e1397. doi:10.7205/MILMED-D-15-00575. PMID 27753588.
- Yarrow JF, McCoy SC, Borst SE (June 2010). "Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity". Steroids. 75 (6): 377–89. doi:10.1016/j.steroids.2010.01.019. PMID 20138077. S2CID 205253265.
- Metzler M (April 1989). "Metabolism of some anabolic agents: toxicological and analytical aspects". J. Chromatogr. 489 (1): 11–21. doi:10.1016/s0378-4347(00)82880-7. PMID 2745641.
- Yarrow JF, Conover CF, McCoy SC, Lipinska JA, Santillana CA, Hance JM, Cannady DF, VanPelt TD, Sanchez J, Conrad BP, Pingel JE, Wronski TJ, Borst SE (April 2011). "17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate". Am. J. Physiol. Endocrinol. Metab. 300 (4): E650–60. doi:10.1152/ajpendo.00440.2010. PMC 6189634. PMID 21266670.
- Spranger B, Metzler M (April 1991). "Disposition of 17 beta-trenbolone in humans". J. Chromatogr. 564 (2): 485–92. doi:10.1016/0378-4347(91)80517-g. PMID 1874853.
- Advances in Agronomy. Elsevier. 13 April 2007. pp. 16–. ISBN 978-0-08-048819-6.
- Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH (December 2000). "Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor". APMIS. 108 (12): 838–46. doi:10.1111/j.1600-0463.2000.tb00007.x. PMID 11252818. S2CID 22776408.
- Delettré J, Mornon JP, Lepicard G, Ojasoo T, Raynaud JP (January 1980). "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.