RU-57073
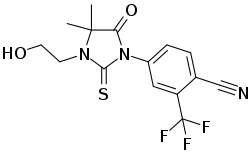
RU-57073 is a nonsteroidal antiandrogen which was never marketed.[1][2][3] It shows 163% of the affinity of testosterone for the androgen receptor and negligible affinity for other steroid hormone receptors.[1][2]
 | |
| Clinical data | |
|---|---|
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C15H14F3N3O2S |
| Molar mass | 357.35 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Curr. Med. Chem. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, Gofflo D, Gaillard-Kelly M, Philibert D (January 1994). "Non-steroidal antiandrogens: synthesis and biological profile of high-affinity ligands for the androgen receptor". J. Steroid Biochem. Mol. Biol. 48 (1): 111–9. doi:10.1016/0960-0760(94)90257-7. PMID 8136296. S2CID 31404295.
- Münster U, Nakamura C, Haberland A, Jores K, Mehnert W, Rummel S, Schaller M, Korting HC, Zouboulis CC, Blume-Peytavi U, Schäfer-Korting M (January 2005). "RU 58841-myristate--prodrug development for topical treatment of acne and androgenetic alopecia". Pharmazie. 60 (1): 8–12. PMID 15700772.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.