BMS-564,929
BMS-564,929 is an investigational selective androgen receptor modulator (SARM) which is being developed by Bristol-Myers Squibb for treatment of the symptoms of age-related decline in androgen levels in men ("andropause"). These symptoms may include depression, loss of muscle mass and strength, reduction in libido and osteoporosis. Treatment with exogenous testosterone is effective in counteracting these symptoms but is associated with a range of side effects, the most serious of which is enlargement of the prostate gland, which can lead to benign prostatic hypertrophy and even prostate cancer. This means there is a clinical need for selective androgen receptor modulators, which produce anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in the prostate.[1]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.303 |
| Chemical and physical data | |
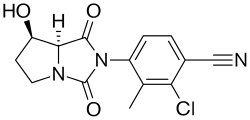
| Formula | C14H12ClN3O3 |
| Molar mass | 305.72 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
BMS-564,929 is one such compound currently in early human clinical trials, which is an orally active, potent and selective agonist for androgen receptors (Ki 2.1nM, 20x functional selectivity for muscle tissue over prostate) and in studies on castrated rats it was shown to counteract decrease in muscle mass over time, and at higher doses even increased muscle mass, without significantly affecting prostate tissue.[2] It does however vastly reduce luteinizing hormone levels, it being an astonishing 33× more suppressive compound than testosterone, which may be a problem in human clinical use.[3]
Selective androgen receptor modulators may also be used by athletes to assist in training and increase physical stamina and fitness, potentially producing effects similar to anabolic steroids but with significantly fewer side effects. For this reason, SARMs have already been banned by the World Anti-Doping Agency since January 2008 despite no drugs from this class yet being in clinical use, and blood tests for all known SARMs are currently being developed.[4][5]
References
- Gao W, Dalton JT (March 2007). "Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs)". Drug Discovery Today. 12 (5–6): 241–8. doi:10.1016/j.drudis.2007.01.003. PMC 2072879. PMID 17331889.
- Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek SR, et al. (January 2007). "Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats". Endocrinology. 148 (1): 4–12. doi:10.1210/en.2006-0843. PMID 17008401.
- Gao W, Dalton JT (February 2007). "Ockham's razor and selective androgen receptor modulators (SARMs): are we overlooking the role of 5alpha-reductase?". Molecular Interventions. 7 (1): 10–3. doi:10.1124/mi.7.1.3. PMC 2040232. PMID 17339601.
- Thevis M, Kohler M, Schlörer N, Kamber M, Kühn A, Linscheid MW, Schänzer W (May 2008). "Mass spectrometry of hydantoin-derived selective androgen receptor modulators". Journal of Mass Spectrometry. 43 (5): 639–50. Bibcode:2008JMSp...43..639T. doi:10.1002/jms.1364. PMID 18095383.
- Thevis M, Kohler M, Thomas A, Maurer J, Schlörer N, Kamber M, Schänzer W (May 2008). "Determination of benzimidazole- and bicyclic hydantoin-derived selective androgen receptor antagonists and agonists in human urine using LC-MS/MS". Analytical and Bioanalytical Chemistry. 391 (1): 251–61. doi:10.1007/s00216-008-1882-6. PMID 18270691. S2CID 206899531.