Pyrilutamide
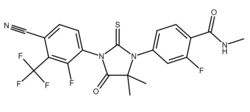
Pyrilutamide (developmental code name KX-826) is a nonsteroidal antiandrogen (NSAA) – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR) – which is under development by Suzhou Kintor Pharmaceuticals, inc., a subsidiary of Kintor Pharmaceutical Limited, for the potential treatment of androgenetic alopecia in males and females in China and the United States of America. [1][2]
 | |
| Clinical data | |
|---|---|
| Other names | Pyrilutamide; KX-826 |
| Routes of administration | Topical |
| Drug class | Nonsteroidal antiandrogen |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| Chemical and physical data | |
| Formula | C21H15F5N4O2S |
| Molar mass | 482.43 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Development
Pyrilutamide successfully completed phase II clinical trials in China and is currently in phase II clinical trials in the U.S for the potential treatment of androgenetic alopecia in males. It is currently in phase II clinical trials in China for the potential treatment of androgenetic alopecia in females. The drug is about to enter phase III clinical trials in China which will be conducted over 24 weeks across more than 20 sites in China with a sample size of 416.
The primary endpoint is the change from baseline in non-vellus target area hair count (TAHC) at the end of week 24. The drug will be dosed at 5mg (0.5%) per patient per day in the trial which is expected to start in Q1 2022.[3]
Adverse effects
Pyrilutamide is generally well-tolerated. The most common adverse event is contact dermatitis.[4]
Pharmacology

Pharmacodynamics
Pyrilutamide binds to the androgen receptor with a very high affinity with an IC50 of 0.28nM. [2] Reference drug Bicalutamide had an IC50 of 3.1nM. [2]
Pharmacokinetics
In Phase I clinical trials, Pyrilutamide and metabolite KX-982's systemic absorption was evaluated across different doses of 0.5, 2, 6, 12, and 24 mg/body/day.
References
- "Pyrilutamide - Suzhou Kintor Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG.
- CA patent 2829322, Tong Y, "Substituted Thioimidazolidinone Androgen Receptor Antagonists and Uses Thereof", published 2012-03-08, issued 2017-01-10, assigned to Suzhou Kintor Pharmaceuticals, Inc
- "Kintor Pharma Announces the Primary Endpoint of Phase II Clinical Study for KX-826's Treatment of Androgenetic Alopecia Was Met".
- "Kintor Pharmaceutical (9939 HK) Specializing in AR-related innovative therapies" (PDF).