Mesterolone
Mesterolone, sold under the brand name Proviron among others, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels.[1][2] It has also been used to treat male infertility, although this use is controversial.[1][3][4] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Proviron, others |
| Other names | NSC-75054; SH-60723; SH-723; 1α-Methyl-4,5α-dihydrotestosterone; 1α-Methyl-DHT; 1α-Methyl-5α-androstan-17β-ol-3-one |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 3% |
| Protein binding | 98% (40% to Albumin, 58% to SHBG) |
| Metabolism | Liver |
| Elimination half-life | 12-13 hours |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.397 |
| Chemical and physical data | |
| Formula | C20H32O2 |
| Molar mass | 304.474 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Side effects of mesterolone include symptoms of masculinization like acne, scalp hair loss, increased body hair growth, voice changes, and increased sexual desire.[1] It has no risk of liver damage.[1][2] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1][5] It has strong androgenic effects and weak anabolic effects, which make it useful for producing masculinization.[1] The drug has no estrogenic effects.[1][2]
Mesterolone was first described by 1966[6] and introduced for medical use by 1967.[7][8] In addition to its medical use, mesterolone has been used to improve physique and performance, although it is not commonly used for such purposes due to its weak anabolic effects.[1] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[1][9]
Medical uses
Mesterolone is used in the treatment of androgen deficiency in male hypogonadism, anemia, and to support male fertility among other indications.[1][10][11] It has also been used to treat delayed puberty in boys.[12] Because it lacks estrogenic effects, mesterolone may be indicated for treating cases of androgen deficiency in which breast tenderness or gynecomastia is also present.[13] The drug is described as a relatively weak androgen with partial activity and is rarely used for the purpose of androgen replacement therapy, but is still widely used in medicine.[1][11][14][2]
Mesterolone is used in androgen replacement therapy at a dosage of 50 to 100 mg 2 to 3 times per day.[15]
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosteronea | – | Tablet | 400–800 mg/day (in divided doses) |
| Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg/2–4x day (with meals) | |
| Methyltestosteroneb | Android, Metandren, Testred | Tablet | 10–50 mg/day | |
| Fluoxymesteroneb | Halotestin, Ora-Testryl, Ultandren | Tablet | 5–20 mg/day | |
| Metandienoneb | Dianabol | Tablet | 5–15 mg/day | |
| Mesteroloneb | Proviron | Tablet | 25–150 mg/day | |
| Sublingual | Testosteroneb | Testoral | Tablet | 5–10 mg 1–4x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 10–30 mg/day | |
| Buccal | Testosterone | Striant | Tablet | 30 mg 2x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 5–25 mg/day | |
| Transdermal | Testosterone | AndroGel, Testim, TestoGel | Gel | 25–125 mg/day |
| Androderm, AndroPatch, TestoPatch | Non-scrotal patch | 2.5–15 mg/day | ||
| Testoderm | Scrotal patch | 4–6 mg/day | ||
| Axiron | Axillary solution | 30–120 mg/day | ||
| Androstanolone (DHT) | Andractim | Gel | 100–250 mg/day | |
| Rectal | Testosterone | Rektandron, Testosteronb | Suppository | 40 mg 2–3x/day |
| Injection (IM or SC) | Testosterone | Andronaq, Sterotate, Virosterone | Aqueous suspension | 10–50 mg 2–3x/week |
| Testosterone propionateb | Testoviron | Oil solution | 10–50 mg 2–3x/week | |
| Testosterone enanthate | Delatestryl | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Xyosted | Auto-injector | 50–100 mg 1x/week | ||
| Testosterone cypionate | Depo-Testosterone | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Testosterone isobutyrate | Agovirin Depot | Aqueous suspension | 50–100 mg 1x/1–2 weeks | |
| Testosterone phenylacetateb | Perandren, Androject | Oil solution | 50–200 mg 1x/3–5 weeks | |
| Mixed testosterone esters | Sustanon 100, Sustanon 250 | Oil solution | 50–250 mg 1x/2–4 weeks | |
| Testosterone undecanoate | Aveed, Nebido | Oil solution | 750–1,000 mg 1x/10–14 weeks | |
| Testosterone buciclatea | – | Aqueous suspension | 600–1,000 mg 1x/12–20 weeks | |
| Implant | Testosterone | Testopel | Pellet | 150–1,200 mg/3–6 months |
| Notes: Men produce about 3 to 11 mg testosterone per day (mean 7 mg/day in young men). Footnotes: a = Never marketed. b = No longer used and/or no longer marketed. Sources: See template. | ||||
Non-medical uses
Mesterolone has been used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.[1]
Side effects
Side effects of mesterolone include virilization among others.[1]
Pharmacology
Pharmacodynamics
Like other AAS, mesterolone is an agonist of the androgen receptor (AR).[1] Mesterolone is described as a very poor anabolic agent due to inactivation by 3α-hydroxysteroid dehydrogenase (3α-HSD) in skeletal muscle tissue, similarly to DHT and mestanolone (17α-methyl-DHT).[1] In contrast, testosterone is a very poor substrate for 3α-HSD, and so is not similarly inactivated in skeletal muscle.[1] Because of its lack of potentiation by 5α-reductase in "androgenic" tissues and its inactivation by 3α-HSD in skeletal muscle, mesterolone is relatively low in both its androgenic potency and its anabolic potency.[1] However, it does still show a greater ratio of anabolic activity to androgenic activity relative to testosterone.[1]
Mesterolone is not a substrate for 5α-reductase, as it is already 5α-reduced, and hence is not potentiated in so-called "androgenic" tissues such as the skin, hair follicles, and prostate gland.[1]
Mesterolone is not a substrate for aromatase, and so cannot be converted into an estrogen.[1] As such, it has no propensity for producing estrogenic side effects such as gynecomastia and fluid retention.[1] It also has no progestogenic activity.[1]
Because mesterolone is not 17α-alkylated, it has little or no potential for hepatotoxicity.[1] However, its risk of deleterious effects on the cardiovascular system is comparable to that of several other oral AAS.[1]
Pharmacokinetics
The C1α methyl group of mesterolone inhibits its hepatic metabolism and thereby confers significant oral activity, although its oral bioavailability is still much lower than that of 17α-alkylated AAS.[1] In any case, mesterolone is one of the few non-17α-alkylated AAS that is active with oral ingestion.[1] Uniquely among AAS, mesterolone has very high affinity for human serum sex hormone-binding globulin (SHBG), about 440% that of DHT in one study and 82% of that of DHT in another study.[16][1][17] As a result, it may displace endogenous testosterone from SHBG and thereby increase free testosterone concentrations, which may in part be involved in its effects.[1]
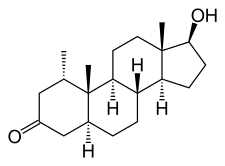
Chemistry
Mesterolone, also known as 1α-methyl-4,5α-dihydrotestosterone (1α-methyl-DHT) or as 1α-methyl-5α-androstan-17β-ol-3-one, is a synthetic androstane steroid and derivative of DHT.[18][19][1] It is specifically DHT with a methyl group at the C1α position.[18][19][1] Closely related AAS include metenolone and its esters metenolone acetate and metenolone enanthate.[18][19][1] The antiandrogen rosterolone (17α-propylmesterolone) is also closely related to mesterolone.[20]
History
Mesterolone was developed in the 1960s[21] and was first described by 1966.[6][22][23][24] It was introduced for medical use by Schering under the brand name Proviron by 1967.[7][8] The well-established brand name Proviron had previously been used by Schering for testosterone propionate starting in 1936.[25] Following the introduction of mesterolone as Proviron, Schering continued to market testosterone propionate under the brand name Testoviron.[25] A number of sources incorrectly state that mesterolone was synthesized or introduced for medical use in 1934.[21][1][26][27]
Society and culture
Generic names
Mesterolone is the generic name of the drug and its INN, USAN, BAN, and DCIT, while mestérolone is its DCF.[18][19][28][29]
Availability
Mesterolone is available widely throughout the world, including in the United Kingdom, Australia, and South Africa, as well as many non-English-speaking countries.[19][29] It is not available in the United States, Canada, or New Zealand.[19][29] The drug has never been marketed in the United States.[26]
Legal status
Mesterolone, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act and a schedule IV controlled substance in Canada under the Controlled Drugs and Substances Act.[9][30]
Research
In one small scale clinical trial of depressed patients, an improvement of symptoms which included anxiety, lack of drive and desire was observed.[31] In patients with dysthymia, unipolar, and bipolar depression significant improvement was observed.[31] In this series of studies, mesterolone lead to a significant decrease in luteinizing hormone and testosterone levels.[31] In another study, 100 mg mesterolone cipionate was administered twice monthly.[32] With regards to plasma testosterone levels, there was no difference between the treated versus untreated group, and baseline luteinizing hormone levels were minimally affected.[32]
References
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 641–. ISBN 978-0-9828280-1-4.
- E. Nieschlag; H. M. Behre (1 April 2004). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 411–. ISBN 978-1-139-45221-2.
- T.B. Hargreave (6 December 2012). Male Infertility. Springer Science & Business Media. pp. 398–399. ISBN 978-1-4471-1029-3.
- Larry I. Lipshultz; Stuart S. Howards; Craig S. Niederberger (24 September 2009). Infertility in the Male. Cambridge University Press. pp. 445–446. ISBN 978-0-521-87289-8.
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Behre, H.M.; Wang, C.; Handelsman, D.J.; Nieschlag, E.; Nieschlag, E.; Behre, H. M.; Nieschlag, S. (2004). "Pharmacology of testosterone preparations". Testosterone. pp. 405–444. doi:10.1017/CBO9780511545221.015. ISBN 9780521833806.
- Rausch-Stroomann JG, Petry R, Hienz HA (1967). "The influence of mesterolone on testicular function". Research on Steroids. Pergamon. 3: 181–184.
- Tausk, M. (1968). "Practically Applicable Results of Twenty Years of Research in Endocrinology". Prog Drug Res. 12: 137–164. doi:10.1007/978-3-0348-7065-8_3. ISBN 978-3-0348-7067-2. PMID 4307936.
- Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- Gautam N. Allahbadia; Rita Basuray Das (12 November 2004). The Art and Science of Assisted Reproductive Techniques. CRC Press. pp. 824–. ISBN 978-0-203-64051-7.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1186–. ISBN 978-0-7817-1750-2.
- I. Hart; R.W. Newton (6 December 2012). Endocrinology. Springer Science & Business Media. pp. 119–. ISBN 978-94-010-9298-2.
- Corona G, Rastrelli G, Vignozzi L, Maggi M (2012). "Emerging medication for the treatment of male hypogonadism". Expert Opin Emerg Drugs. 17 (2): 239–59. doi:10.1517/14728214.2012.683411. PMID 22612692. S2CID 22068249.
- Nieschlag E, Behre HM, Bouchard P, et al. (2004). "Testosterone replacement therapy: current trends and future directions". Hum. Reprod. Update. 10 (5): 409–19. doi:10.1093/humupd/dmh035. PMID 15297434.
- Rastrelli, G.; Reisman, Y.; Ferri, S.; Prontera, O.; Sforza, A.; Maggi, M.; Corona, G. (2019). "Testosterone Replacement Therapy". Sexual Medicine. pp. 79–93. doi:10.1007/978-981-13-1226-7_8. ISBN 978-981-13-1225-0.
- Saartok T, Dahlberg E, Gustafsson JA (1984). "Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin". Endocrinology. 114 (6): 2100–6. doi:10.1210/endo-114-6-2100. PMID 6539197.
- Pugeat MM, Dunn JF, Nisula BC (July 1981). "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. doi:10.1210/jcem-53-1-69. PMID 7195405.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 775–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 656–. ISBN 978-3-88763-075-1.
- Brooks, J. R.; Primka, R. L.; Berman, C; Krupa, D. A.; Reynolds, G. F.; Rasmusson, G. H. (1991). "Topical anti-androgenicity of a new 4-azasteroid in the hamster". Steroids. 56 (8): 428–33. doi:10.1016/0039-128x(91)90031-p. PMID 1788861. S2CID 21500107.
- Malcolm Carruthers (2006). Androgen Deficiency in the Adult Male: Causes, Diagnosis and Treatment. CRC Press. pp. 137–178. ISBN 978-0-367-80018-5.
- Neumann F, Wiechert R, Kramer M, Raspé G (April 1966). "[Experimental animal studies with a new androgen--mesterolone (1-alpha-methyl-5-alpha-androstan-17-beta-ol-one)]". Arzneimittelforschung (in German). 16 (4): 455–8. PMID 6014248.
- Laschet, U.; Niermann, H.; Laschet, L.; Paarmann, H. F. (1967). "Mesterolone, a potent oral active androgen without gonadotropin inhibition". Acta Endocrinologica. 56 (1_Suppl): S55. doi:10.1530/acta.0.056S055. ISSN 0804-4643.
- Tausk M (1968). "Practically applicable results of twenty years of research in endocrinology". Prog Drug Res. 12: 137–64. doi:10.1007/978-3-0348-7065-8_3. ISBN 978-3-0348-7067-2. PMID 4307936.
- Nieschlag, Eberhard; Nieschlag, Susan (2017). "The History of Testosterone and The Testes: From Antiquity to Modern Times". Testosterone. pp. 1–19. doi:10.1007/978-3-319-46086-4_1. ISBN 978-3-319-46084-0.
- Alexandre Hohl (6 April 2017). Testosterone: From Basic to Clinical Aspects. Springer. pp. 204–. ISBN 978-3-319-46086-4.
- Kalinchenko, Svetlana; Tyuzikov, Igor; Mskhalaya, George; Tishova, Yulia (2017). "Testosterone Therapy: Oral Androgens". Testosterone. pp. 203–224. doi:10.1007/978-3-319-46086-4_10. ISBN 978-3-319-46084-0.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 176–177. ISBN 978-94-011-4439-1.
- "Mesterolone".
- Linda Lane Lilley; Julie S. Snyder; Shelly Rainforth Collins (5 August 2016). Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. pp. 50–. ISBN 978-1-77172-066-3.
- Itil TM, Michael ST, Shapiro DM, Itil KZ (June 1984). "The effects of mesterolone, a male sex hormone in depressed patients (a double blind controlled study)". Methods Find Exp Clin Pharmacol. 6 (6): 331–7. PMID 6431212.
- Kövary PM, Lenau H, Niermann H, Zierden E, Wagner H (May 1977). "Testosterone levels and gonadotrophins in Klinefelter's patients treated with injections of mesterolone cipionate". Arch Dermatol Res. 258 (3): 289–94. doi:10.1007/bf00561132. PMID 883846. S2CID 1222130.
External links
Further reading
- Morrison, Mary Chase (2000). Hormones, Gender and the Aging Brain: The Endocrine Basis of Geriatric Psychiatry. Cambridge, UK: Cambridge University Press. p. 134. ISBN 0-521-65304-5.