Paroxypropione
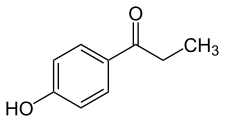
Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed.[1][2][3][4] It was first synthesized in 1902.[1] The antigonadotropic properties of the drug were discovered in 1951[3] and it entered clinical use shortly thereafter.[5]
 | |
| Clinical data | |
|---|---|
| Trade names | Frenantol, Frenormon, Hypophenon, Paroxon, Possipione, Profenone, others |
| Other names | Paraoxypropiophenone; H-365; NSC-2834; 4'-Hydroxypropiophenone; Ethyl p-hydroxyphenyl ketone; p-Propionylphenol; Paroxypropiophenone; Parahydroxypropiophenone; PHP |
| Drug class | Nonsteroidal estrogen; Antigonadotropin |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.676 |
| Chemical and physical data | |
| Formula | C9H10O2 |
| Molar mass | 150.177 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pharmacology
Pharmacodynamics
Paroxypropione is closely related structurally to p-hydroxybenzoic acid and parabens such as methylparaben, and also bears a close resemblance to diethylstilbestrol (which, in fact, produces paroxypropione as an active metabolite)[6][7] and alkylphenols like nonylphenol, all of which are also estrogens.[8][9] The drug possesses relatively low affinity for the estrogen receptor[4] and must be given at high dosages to achieve significant estrogenic and antigonadotropic effects, for instance, 0.8 to 1.6 g/day.[10][11] It possesses 0.1% of the estrogenic activity and less than 0.5% of the antigonadotropic potency of estrone.[12]
Chemistry
Synthesis
The highest reported yield, approximately 96%, is from the between phenol and propionyl chloride.[13] The mechanism is likely to involve initial esterification to give phenyl propionate, which then undergoes a Fries rearrangement.
Derivatives
Paroxypropione is a precursor in the chemical synthesis of diethylstilbestrol and dienestrol.[14][15]
Society and culture
Names
Brand names Frenantol, Frenormon, Hypophenon, Paroxon, Possipione, Profenone, numerous others; former developmental code name NSC-2834), also known as paroxypropiophenone (P.O.P.) or 4'-hydroxypropiophenone.
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 662–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 796–. ISBN 978-3-88763-075-1.
- Paulsen CA, Mortimore GE, Heller CG (August 1951). "The pituitary action and estrogenic effect of parahydroxy-propiophenone". The Journal of Clinical Endocrinology and Metabolism. 11 (8): 892–4. doi:10.1210/jcem-11-8-892. PMID 14861299.
- Mombelli E (January 2012). "Evaluation of the OECD (Q)SAR Application Toolbox for the profiling of estrogen receptor binding affinities". SAR and QSAR in Environmental Research. 23 (1–2): 37–57. doi:10.1080/1062936X.2011.623325. PMID 22014213. S2CID 19751228.
- Buu-Hoi NP, Xuong ND, Lavit D (1953). "Fluorine-containing analogs of 4-hydroxypropiophenone". The Journal of Organic Chemistry. 18 (8): 910–915. doi:10.1021/jo50014a002. ISSN 0022-3263.
- Chambers PL, Günzel P (12 March 2013). Mechanism of Toxic Action on Some Target Organs: Drugs and Other Substances. Springer Science & Business Media. pp. 276–. ISBN 978-3-642-67265-1.
- Gottschlich R, Metzler M (January 1979). "High-pressure, reverse-phase partition chromatograhy separation of diethylstilbestrol metabolites and analogs". Analytical Biochemistry. 92 (1): 199–202. doi:10.1016/0003-2697(79)90645-6. PMID 426279.
- Jones RE, Lopez KH (28 September 2013). Human Reproductive Biology. Academic Press. pp. 46–. ISBN 978-0-12-382185-0.
- Pugazhendhi D, Pope GS, Darbre PD (2005). "Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines". Journal of Applied Toxicology. 25 (4): 301–9. doi:10.1002/jat.1066. PMID 16021681. S2CID 12342018.
- De Vega R (1955). "Protein breakdown before and after operations. Influence of growth hormone and of inhibitors of the pituitary adrenal system". Cirug., Ginecol. Urol. 9: 289–326.
- Bussolati C, de Carneri I, Castellino S, Marinoni V, Sperzani GL (July 1967). "Treatment of experimental and clinical schistosomiasis with hormonal inhibitors of ovulation". The American Journal of Tropical Medicine and Hygiene. 16 (4): 497–9. doi:10.4269/ajtmh.1967.16.497. PMID 5006470.
- Scott CC, Kroc RL, Stasilli NR (June 1952). "Metabolic and toxicity studies on parahydroxypropiophenone". Endocrinology. 50 (6): 607–11. doi:10.1210/endo-50-6-607. PMID 12980070.
- Murashige R, Hayashi Y, Ohmori S, Torii A, Aizu Y, Muto Y, Murai Y, Oda Y, Hashimoto M (2011). "Comparisons of O-acylation and Friedel–Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonicacid: effective synthesis of optically active homotyrosines". Tetrahedron. 67 (3): 641–649. doi:10.1016/j.tet.2010.11.047. hdl:2115/44794.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1286, 1290. ISBN 978-0-8155-1856-3.
- Szmant HH (1989). Organic Building Blocks of the Chemical Industry. John Wiley & Sons. pp. 532–. ISBN 978-0-471-85545-3.
- Maconi G (June 1955). "[Hydroxypropiophenone in the therapy of metastases of carcinoma of the breast]" [Hydroxypropiophenone in the therapy of metastases of carcinoma of the breast]. Il Farmaco; Edizione Pratica (in Italian). 10 (6): 291–9. PMID 13241536.
- Stoll BA (August 1956). "P-hydroxypropiophenone for advanced breast cancer: a preliminary report". The Medical Journal of Australia. 43 (5): 181–3. doi:10.5694/j.1326-5377.1956.tb56562.x. PMID 13358357. S2CID 22364847.
- Grapulin G (June 1967). "[Experience with paraoxypropiophenone (Frenantol) in the treatment of dysplasias and metastasized carcinoma of the breast]" [Experience with paraoxypropiophenone (Frenantol) in the treatment of dysplasias and metastasized carcinoma of the breast]. Chirurgia Italiana (in Italian). 19 (3): 306–12. PMID 5188348.
Further reading
- Gustavo RP (July 1958). "[Anti-gonadotropic action of possipione]" [Anti-gonadotropic action of possipione]. Quaderni di Clinica Ostetrica e Ginecologica (in Italian). 13 (7): 307–15. PMID 13579130.