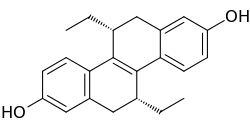
(R,R)-Tetrahydrochrysene
(R,R)-Tetrahydrochrysene ((R,R)-THC) is a drug used to study the estrogen receptors (ERs) in scientific research. It is an ERβ antagonist and an ERα agonist with 10-fold higher affinity for ERβ relative to ERα.[1][2] (R,R)-THC is a silent antagonist of ERβ,[3] and, uniquely relative to other known ERβ antagonists, a passive antagonist of the receptor.[2]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C22H24O2 |
| Molar mass | 320.432 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
(S,S)-Tetrahydrochrysene ((S,S)-THC) also binds to the ERs, but in contrast to (R,R)-THC, (S,S)-THC is an agonist of both ERα and ERβ and has 20-fold lower affinity for ERβ relative to (R,R)-THC.[3]
See also
- Propylpyrazoletriol (PPT)
- PHTPP
- Methylpiperidinopyrazole (MPP)
- Diarylpropionitrile (DPN)
- Prinaberel (ERB-041)
- Liquiritigenin
- Menerba
- 2,8-DHHHC
- Chrysene
References
- Ying Chen (2008). The Role of Steroids in the Regulation of Oocyte Cyst Breakdown and Primordial Follicle Assembly in the Neonatal Mouse Ovary. pp. 101–. ISBN 978-0-549-74620-1.
- Shiau AK, Barstad D, Radek JT, Meyers MJ, Nettles KW, Katzenellenbogen BS, Katzenellenbogen JA, Agard DA, Greene GL (2002). "Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism". Nat. Struct. Biol. 9 (5): 359–64. doi:10.1038/nsb787. PMID 11953755. S2CID 452305.
- Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS (1999). "Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta". Endocrinology. 140 (2): 800–4. doi:10.1210/endo.140.2.6480. PMID 9927308.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.