SERBA-2
SERBA-2, short for selective estrogen receptor beta agonist-2, is a synthetic, nonsteroidal estrogen which acts as a selective ERβ agonist.[2][3] For the ERα and ERβ, SERBA-2 has affinities (Ki) of 14.5 nM and 1.54 nM, efficacies of 85% and 100%, and EC50 values of 85 nM and 3.61 nM, respectively, demonstrating 9-fold binding selectivity and 11-fold functional selectivity for the ERβ over the ERα.[2][3][4][1] An enantiomer of SERBA-2, erteberel (SERBA-1), is more potent and selective in comparison and is under development for the treatment of schizophrenia.[5][2][3][1]
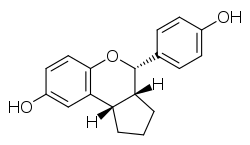 | |
| Clinical data | |
|---|---|
| Other names | (2S,3R,4S)-SERBA[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H18O3 |
| Molar mass | 282.339 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Paterni I, Bertini S, Granchi C, Macchia M, Minutolo F (2013). "Estrogen receptor ligands: a patent review update". Expert Opin Ther Pat. 23 (10): 1247–71. doi:10.1517/13543776.2013.805206. PMID 23713677. S2CID 6259593.
- Norman BH, Dodge JA, Richardson TI, Borromeo PS, Lugar CW, Jones SA, Chen K, Wang Y, Durst GL, Barr RJ, Montrose-Rafizadeh C, Osborne HE, Amos RM, Guo S, Boodhoo A, Krishnan V (2006). "Benzopyrans are selective estrogen receptor beta agonists with novel activity in models of benign prostatic hyperplasia". J. Med. Chem. 49 (21): 6155–7. doi:10.1021/jm060491j. PMID 17034120.
- Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA (2011). "Estrogen receptor β ligands: recent advances and biomedical applications". Med Res Rev. 31 (3): 364–442. doi:10.1002/med.20186. PMID 19967775. S2CID 3841877.
- Lóránd T, Vigh E, Garai J (2010). "Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens". Curr. Med. Chem. 17 (30): 3542–74. doi:10.2174/092986710792927813. PMID 20738246.
- "Erteberel - AdisInsight". adisinsight.springer.com. Archived from the original on 31 December 2016. Retrieved 15 January 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.