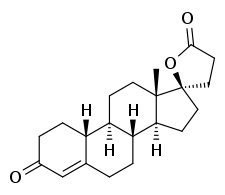
SC-8109
SC-8109 is a steroidal antimineralocorticoid of the spirolactone group which was never marketed.[1][2] It is a potent antagonist of the mineralocorticoid receptor and is more potent than the related drug SC-5233 (of which SC-8109 is the 19-nor analogue).[1][3] However, SC-8109 was found to have relatively low oral bioavailability and potency,[1][4] though it nonetheless produced a mild diuretic effect in patients with congestive heart failure.[2] Spironolactone (SC-9420; Aldactone), another spirolactone, followed and had both good oral bioavailability and potency, and was the first antimineralocorticoid to be marketed.[1][5]
 | |
| Clinical data | |
|---|---|
| Other names | 19-Norspirolactone; 19-Nor-17α-(2-carboxyethyl)testosterone γ-lactone; 3-Oxo-17β-hydroxyestr-4-ene-17-propanoic acid lactone; 17-Hydroxy-3-oxo-19-Nor-17α-pregn-4-ene-21-carboxylic acid γ-lactone |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H28O3 |
| Molar mass | 328.452 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
In addition to its antimineralocorticoid activity, SC-8109 shows potent progestogenic activity, with similar potency relative to that of progesterone.[6] Its analogue, SC-5233, possesses similar but less potent progestogenic activity.[6] In addition, SC-5233 has been assessed and found to possess some antiandrogenic activity, antagonizing the effects of testosterone in animals, and SC-8109 may as well.[7]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 3–10 | <1 | <1 | 3–10 | ? | ? |
| SC-8109 | 191 | 25–50 | <1 | <1 | 15–25 | ? | ? |
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, DEXA for the GR, and aldosterone for the MR. | |||||||
See also
References
- P. J. Bentley (1980). Endocrine Pharmacology: Physiological Basis and Therapeutic Applications. CUP Archive. pp. 160–. ISBN 978-0-521-22673-8.
- E. Buchborn; K. D. Bock (14 December 2013). Diuresis and Diuretics / Diurese und Diuretica: An International Symposium Herrenchiemsee, June 17th–20th, 1959 Sponsored by CIBA / Ein Internationales Symposium Herrenchiemsee, 17.–20. Juni 1959 Veranstaltet mit Unterstützung der CIBA. Springer-Verlag. pp. 224, 261. ISBN 978-3-642-49716-2.
- Hans H. Ussing; Poul Kruhoffer; Hess J. Thaysen; N.H. Thorn (8 March 2013). The Alkali Metal Ions in Biology: I. The Alkali Metal Ions in Isolated Systems and Tissues. II. The Alkali Metal Ions in the Organism. Springer Science & Business Media. pp. 418–. ISBN 978-3-642-49246-4.
- Milan L. Brandon (1 January 1962). Corticosteroids in medical practice. Thomas. ISBN 9780398002152.
- Dennis V. Cokkinos (6 November 2014). Introduction to Translational Cardiovascular Research. Springer. pp. 61–. ISBN 978-3-319-08798-6.
- Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 371–. ISBN 978-1-4832-7299-3.
- Kagawa CM, Sturtevant FM, Van Arman CG (1959). "Pharmacology of a new steroid that blocks salt activity of aldosterone and desoxycorticosterone". J. Pharmacol. Exp. Ther. 126 (2): 123–30. PMID 13665517.
[SC-5233] (total dose of 5 mg/rat) partially blocked the effects of testosterone propionate on the seminal vesicles and prostate in similar animals.
- Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids". Drug Design. pp. 169–214. doi:10.1016/B978-0-12-060308-4.50010-X. ISBN 9780120603084.