20α-Dihydrodydrogesterone
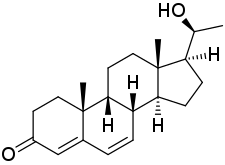
20α-Dihydrodydrogesterone (20α-DHD), also known as 20α-hydroxydydrogesterone, as well as 20(S)-hydroxy-9β,10α-pregna-4,6-dien-3-one, is a progestin and the major active metabolite of dydrogesterone.[2][1][3] It appears that dydrogesterone is a prodrug of 20α-DHD, as it is largely transformed into this metabolite when given orally in humans.[3] 20α-DHD has progestogenic activity similarly to dydrogesterone, but is far less potent in comparison.[3]
 | |
| Clinical data | |
|---|---|
| Other names | 20α-DHD; 20α-Hydroxydydrogesterone; 20(S)-Hydroxy-9β,10α-pregna-4,6-dien-3-one |
| Drug class | Progestin; Progestogen |
| Pharmacokinetic data | |
| Elimination half-life | 14–17 hours[1] |
| Excretion | Urine (mainly as glucuronide conjugates) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Bińkowska, Małgorzata; Woroń, Jarosław (2015). "Progestogens in menopausal hormone therapy". Menopausal Review. 14 (2): 134–143. doi:10.5114/pm.2015.52154. ISSN 1643-8876. PMC 4498031. PMID 26327902.
- Olbrich, Matthias; Weigl, Kevin; Kahler, Elke; Mihara, Katsuhiro (2016). "Dydrogesterone metabolism in human liver by aldo-keto reductases and cytochrome P450 enzymes". Xenobiotica. 46 (10): 868–874. doi:10.3109/00498254.2015.1134852. ISSN 0049-8254. PMID 26796435. S2CID 22311056.
- Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, van der Burg B, Böcker C, Husen B (May 2011). "Selectivity and potency of the retroprogesterone dydrogesterone in vitro". Steroids. 76 (6): 607–15. doi:10.1016/j.steroids.2011.02.043. PMID 21376746. S2CID 31609405.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.