Cortifen
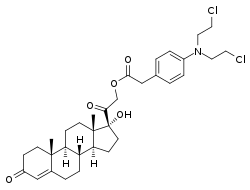
Cortifen, also known as cortiphen or kortifen, as well as fencoron, is a synthetic glucocorticoid corticosteroid and cytostatic antineoplastic agent which was developed in Russia for potential treatment of tumors.[1][2][3] It is a hydrophobic chlorphenacyl nitrogen mustard ester of 11-deoxycortisol (cortodoxone).[1][2][3][4]
 | |
| Clinical data | |
|---|---|
| Other names | Cortiphen; Kortifen; Fencoron; 11-Deoxycortisol 21-(4-(bis(2-chloroethyl)amino)phenyl)acetate; 11-Desoxy-17α-hydroxy-21-[n-di-2(chlorethyl)aminophenyl acetate]corticosterone |
| Drug class | Cytostatic antineoplastic agent; Corticosteroid; Glucocorticoid |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C33H43Cl2NO5 |
| Molar mass | 604.61 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Lagova ND, Kiselev VI, Kurdiumova KN, Sof'ina ZP, Shkodinskaia EN (1989). "[Experimental study of the antitumor properties and mechanism of action of kortifen]". Vopr Onkol (in Russian). 35 (4): 450–6. PMID 2728387.
- Oborotov, A. V.; Smirnova, Z. S.; Klochkova, T. I.; Arzamastsev, A. P. (1999). "Biopharmaceutical investigation of a new medicinal form of the antitumor drug cortifen". Pharmaceutical Chemistry Journal. 33 (10): 540–542. doi:10.1007/BF02508377. ISSN 0091-150X. S2CID 518576.
- Smirnova, Z. S.; Rodionova, Yu. V.; Khalanskii, A. S.; Gershtein, E. S.; Gerasimova, G. K. (1999). "Dependence of antitumor effect of hormonal cytostatic cortifen on expression of glucocorticoid receptors in brain tumor cells". Bulletin of Experimental Biology and Medicine. 127 (3): 299–300. doi:10.1007/BF02433363. ISSN 0007-4888. S2CID 20261670.
- Oborotova NA, Smirnova ZS, Polozkova ZS, Baryshnikov AIu (2002). "[Pharmacological aspects in the development of liposomal medicinal preparations for the internal injection of hydrophobic cytostatics]". Vestn. Akad. Med. Nauk SSSR (in Russian) (1): 42–5. PMID 11882971.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.