Diflucortolone valerate
Diflucortolone valerate (also Nerisone cream/oily cream/ointment, Neriderm ointment, Japanese ジフルコルトロン (Jifurucorutoron) is a corticosteroid rated Class 2 "potent" (100-150 times) in the New Zealand topical steroid system. It is a white to creamy white crystalline powder. It is practically insoluble in water, freely soluble in dichloromethane and in dioxan, sparingly soluble in ether and slightly soluble in methyl alcohol. Chemically, it is a corticosteroid esterified with valeric acid. It is commonly used topically in dermatology. The brand name is Nerisone; its creams come in potencies of 0.1% and 0.3%.
 | |
| Names | |
|---|---|
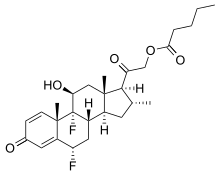
| IUPAC name
6α,9-Difluoro-11β-hydroxy-16α-methyl-3,20-dioxopregna-1,4-dien-21-yl pentanoate | |
| Preferred IUPAC name
2-[(1S,2R,3aS,3bS,5S,9aS,9bR,10S,11aS)-5,9b-Difluoro-10-hydroxy-2,9a,11a-trimethyl-7-oxo-2,3,3a,3b,4,5,7,9a,9b,10,11,11a-dodecahydro-1H-cyclopenta[a]phenanthren-1-yl]-2-oxoethyl pentanoate | |
| Other names
Afusona; Diflucortolone 21-valerate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.056.032 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C27H36F2O5 |
| Molar mass | 478.577 g·mol−1 |
| Melting point | 220 °C (428 °F; 493 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.