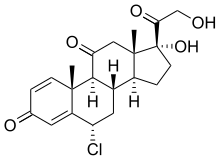
Chloroprednisone
Chloroprednisone is a topical glucocorticoid first reported in 1960.[1] It is a chlorinated derivative of prednisone. The acetate ester prodrug, chloroprednisone 21-acetate, was sold under the brand name Topilan as an anti-inflammatory agent.[2][3]
 | |
| Clinical data | |
|---|---|
| Other names | 6α-Chloro-1,4-pregnadiene-17a,21-diol-3,11,20-trione |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.052.387 |
| Chemical and physical data | |
| Formula | C21H25ClO5 |
| Molar mass | 392.88 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
There is little published about chloroprednisone. This may be due to limited activity topically because the skin lacks the necessary activating enzyme 11-Beta hydroxysteroid dehydrogenase. Systemically, this agent's activity on glucocorticoid receptors may not have competed with agents like fludrocortisone or dexamethasone.
References
- DE 1079042, Batres E, Bowers A, Djerassi C, Kincl FA, Mancera O, Ringold HJ, Rosenkranz J, Zaffaroni A, "6α-Chloro- or 6α-fluoro-1,4-pregnadiene-3,20-diones.", issued 1960.
- Budavari S, ed. (1989). "2157: Chloroprednisone". The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th, centennial ed.). Rahway, N.J., U.S.A.: Merck. ISBN 978-0-911910-28-5.
- Roberts AD (1991). Dictionary of Steroids: Chemical Data, Structures, and Bibliographies. Vol. 1. CRC Press. p. 108.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.