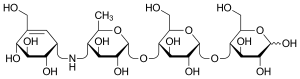
Acarbose
Acarbose (INN)[1][2] is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay (Bayer AG), in North America as Precose (Bayer Pharmaceuticals), and in Canada as Prandase (Bayer AG). It is cheap and popular in China, but not in the U.S. One physician explains the use in the U.S. is limited because it is not potent enough to justify the side effects of diarrhea and flatulence.[3] However, a recent large study concludes "acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes."[4] A possible explanation for the differing opinions is an observation that acarbose is significantly more effective in patients eating a relatively high carbohydrate Eastern diet.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Glucobay, Precose, Prandase |
| Other names | (2R,3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5- {[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl- 5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3- (hydroxymethyl)cyclohex-2-en-1-yl]amino} tetrahydro-2H-pyran-2-yl]oxy}-3,4-dihydroxy- 6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}- 6-(hydroxymethyl)tetrahydro-2H-pyran-2,3,4-triol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extremely low |
| Metabolism | Gastrointestinal tract
glucosidase maltogenic alpha-amylase and cyclomaltodextrinase from gut microbiome |
| Elimination half-life | 2 hours |
| Excretion | Renal (less than 2%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.555 |
| Chemical and physical data | |
| Formula | C25H43NO18 |
| Molar mass | 645.608 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
It is a starch blocker, and inhibits alpha glucosidase, an intestinal enzyme that releases glucose from larger carbohydrates. It is composed of an acarviosin moiety with a maltose at the reducing terminus. Acarbose is also degraded to maltose and acarviosin by the glucosidase cyclomaltodextrinase from gut bacteria Lactobacillus plantarum.[6]
Natural distribution
In nature, acarbose is synthesized by soil bacteria Actinoplanes sp through its precursor valienamine.[7] And acarbose is also degraded by gut bacteria Lactobacillus plantarum and soil bacteria Thermus sp by acarbose degrading glucosidases.
Mechanism of action
Acarbose inhibits enzymes (glycoside hydrolases) needed to digest carbohydrates, specifically, alpha-glucosidase enzymes in the brush border of the small intestines, and pancreatic alpha-amylase. It locks up the enzymes by mimicking the transisition state of the substrate with its amine linkage.[8] However, bacterial alpha-amylases from gut microbiome are able to degrade acarbose.[9][10][11]
Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine, whereas the membrane-bound intestinal alpha-glucosidases hydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the small intestine. Inhibition of these enzyme systems reduces the rate of digestion of complex carbohydrates. Less glucose is absorbed because the carbohydrates are not broken down into glucose molecules. In diabetic patients, the short-term effect of these drug therapies is to decrease current blood glucose levels; the long-term effect is a reduction in HbA1c level.[12] This reduction averages an absolute decrease of 0.7%, which is a decrease of about 10% in typical HbA1c values in diabetes studies.[13]
Combination therapy
The combination of acarbose with metformin results in greater reductions of HbA1c, fasting blood glucose and post-prandial glucose than either agent alone.[14]
Dosing
Since acarbose prevents the digestion of complex carbohydrates, the drug should be taken at the start of main meals (taken with first bite of meal). Moreover, the amount of complex carbohydrates in the meal will determine the effectiveness of acarbose in decreasing postprandial hyperglycemia. Adults may take doses of 25 mg 3 times daily, increasing to 100 mg 3 times a day. However the maximum dose per day is 600 mg.
Side-effects
Since acarbose prevents the degradation of complex carbohydrates into glucose, some carbohydrate will remain in the intestine and be delivered to the colon. In the colon, bacteria digest the complex carbohydrates, causing gastrointestinal side-effects such as flatulence (78% of patients) and diarrhea (14% of patients). Since these effects are dose-related, in general it is advised to start with a low dose and gradually increase the dose to the desired amount. One study found that gastrointestinal side effects decreased significantly (from 50% to 15%) over 24 weeks, even on constant dosing.[15]
If a patient using acarbose has a bout of hypoglycemia, the patient must eat something containing monosaccharides, such as glucose tablets or gel (GlucoBurst, Insta-Glucose, Glutose, Level One) and a doctor should be called. Because acarbose blocks the breakdown of table sugar and other complex sugars, fruit juice or starchy foods will not effectively reverse a hypoglycemic episode in a patient taking acarbose.[16]
Hepatitis has been reported with acarbose use. It usually goes away when the medicine is stopped.[17] Therefore, liver enzymes should be checked before and during use of this medicine.
Life extension
In studies conducted by three independent laboratories by the US National Institute on Aging's intervention testing programme, acarbose was shown to extend the lifespan of female mice by 5% and of male mice by 22%.[18][19]
Metabolism
Acarbose degradation is the unique feature of glycoside hydrolases in gut microbiota, acarbose degrading glucosidase, which hydrolyze acarbose into an acarviosine-glucose and glucose.[11] Human enzymes do transform acarbose: the pancreatic alpha-amylase is able to perform a rearrangement reaction, moving the glucose unit in the "tail" maltose to the "head" of the molecule. Analog drugs with the "tail" glucose removed or flipped to an α(1-6) linkage resist this transformation.[8]
It has been reported that the maltogenic alpha-amylase from Thermus sp. IM6501 (ThMA) and a cyclodextrinase (CDase) from Streptococcus pyogenes could hydrolyse acarbose to glucose and acarviosine-glucose, ThMA can further hydrolyze acarviosine-glucose into acarviosin and glucose.[20][21] A cyclomaltodextrinase (CDase) from gut bacteria Lactobacillus plantarum degraded acarbose via two different modes of action to produce maltose and acarviosin, as well as glucose and acarviosine-glucose, suggest that acarbose resistance is caused by in the human microbiome.[6] The microbiome-derived acarbose kinases are also specific to phosphorylate and inactivate acarbose.[22] The molecular modeling showed the interaction between gut bacterial acarbose degrading glucosidase and human α-amylase.[23]
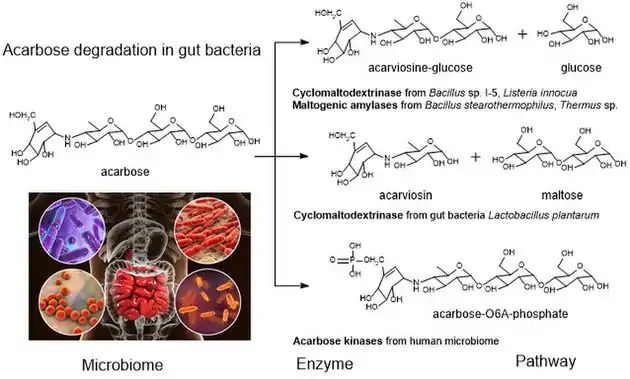
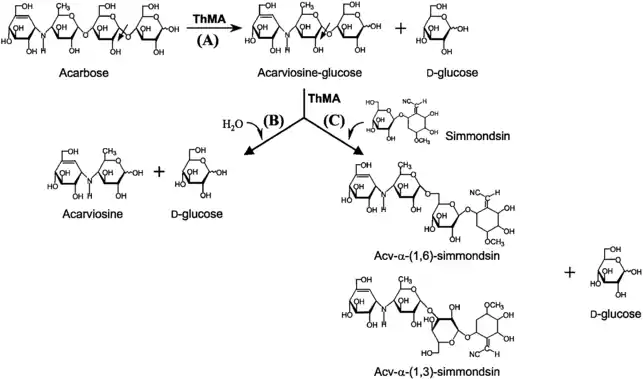
In molecular biology
Acarbose is described chemically as a pseudotetrasaccharide,[24] specifically a maltotetraose mimic inhibitor. As an inhibitor that mimics some natural substrates, it is useful for elucidating the structure of sugar-digesting enzymes, by binding into the same pocket.[25]
References
- Elks J, Ganellin CR (1990). Elks J, Ganellin CR (eds.). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1–. doi:10.1007/978-1-4757-2085-3. ISBN 978-1-4757-2085-3.
- "International Nonproprietary Names for Pharmaceutical Substances. Recommended International Nonproprietary Names (Rec. INN): List 19" (PDF). World Health Organization. 1979. Retrieved 9 November 2016.
- Kresge N (21 November 2011). "China's Thirst for New Diabetes Drugs Threatens Bayer's Lead". Bloomberg Business Week. Archived from the original on 21 November 2011. Retrieved 15 April 2016.
- Zhang W, Kim D, Philip E, Miyan Z, Barykina I, Schmidt B, Stein H (April 2013). "A multinational, observational study to investigate the efficacy, safety and tolerability of acarbose as add-on or monotherapy in a range of patients: the Gluco VIP study". Clinical Drug Investigation. 33 (4): 263–274. doi:10.1007/s40261-013-0063-3. PMID 23435929. S2CID 207483590.
- Zhu Q, Tong Y, Wu T, Li J, Tong N (June 2013). "Comparison of the hypoglycemic effect of acarbose monotherapy in patients with type 2 diabetes mellitus consuming an Eastern or Western diet: a systematic meta-analysis". Clinical Therapeutics. 35 (6): 880–899. doi:10.1016/j.clinthera.2013.03.020. PMID 23602502.
- Jang MU, Kang HJ, Jeong CK, Kang Y, Park JE, Kim TJ (February 2018). "Functional expression and enzymatic characterization of Lactobacillus plantarum cyclomaltodextrinase catalyzing novel acarbose hydrolysis". Journal of Microbiology. 56 (2): 113–118. doi:10.1007/s12275-018-7551-3. PMID 29392561. S2CID 2660911.
- Tsunoda T, Samadi A, Burade S, Mahmud T (June 2022). "Complete biosynthetic pathway to the antidiabetic drug acarbose". Nature Communications. 13 (1): 3455. doi:10.1038/s41467-022-31232-4. PMC 9200736. PMID 35705566.
- Li, Chunmin; Begum, Anjuman; Numao, Shin; Park, Kwan Hwa; Withers, Stephen G.; Brayer, Gary D. (1 March 2005). "Acarbose Rearrangement Mechanism Implied by the Kinetic and Structural Analysis of Human Pancreatic α-Amylase in Complex with Analogues and Their Elongated Counterparts". Biochemistry. 44 (9): 3347–3357. doi:10.1021/bi048334e. PMID 15736945.
- Park KH, Kim MJ, Lee HS, Han NS, Kim D, Robyt JF (December 1998). "Transglycosylation reactions of Bacillus stearothermophilus maltogenic amylase with acarbose and various acceptors". Carbohydrate Research. 313 (3–4): 235–246. doi:10.1016/S0008-6215(98)00276-6. PMID 10209866.
- Oh SW, Jang MU, Jeong CK, Kang HJ, Park JM, Kim TJ (August 2008). "Modulation of hydrolysis and transglycosylation activity of Thermus maltogenic amylase by combinatorial saturation mutagenesis". Journal of Microbiology and Biotechnology. 18 (8): 1401–1407. PMID 18756100.
- Kim TJ, Kim MJ, Kim BC, Kim JC, Cheong TK, Kim JW, Park KH (April 1999). "Modes of action of acarbose hydrolysis and transglycosylation catalyzed by a thermostable maltogenic amylase, the gene for which was cloned from a Thermus strain". Applied and Environmental Microbiology. 65 (4): 1644–1651. Bibcode:1999ApEnM..65.1644K. doi:10.1128/AEM.65.4.1644-1651.1999. PMC 91232. PMID 10103262.
- Drug Therapy in Nursing, 2nd Edition.
- Scheen AJ (September 1998). "Clinical efficacy of acarbose in diabetes mellitus: a critical review of controlled trials". Diabetes & Metabolism. 24 (4): 311–320. PMID 9805641.
- Hedrington MS, Davis SN (December 2019). "Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes". Expert Opinion on Pharmacotherapy. 20 (18): 2229–2235. doi:10.1080/14656566.2019.1672660. PMID 31593486. S2CID 203985605.
- Hoffmann J, Spengler M (December 1997). "Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II Study". The American Journal of Medicine. 103 (6): 483–490. doi:10.1016/S0002-9343(97)00252-0. PMID 9428831.
- "Acarbose". MedlinePlus Drug Information.
- "Acarbose: hepatitis: France, Spain". WHO Pharmaceuticals Newsletter. 1999. Archived from the original on October 15, 2009.
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. (April 2014). "Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males". Aging Cell. 13 (2): 273–282. doi:10.1111/acel.12170. PMC 3954939. PMID 24245565.
- Ladiges W, Liggitt D (2017). "Testing drug combinations to slow aging". Pathobiology of Aging & Age Related Diseases. 8 (1): 1407203. doi:10.1080/20010001.2017.1407203. PMC 5706479. PMID 29291036.
- Jang MU, Kang HJ, Jeong CK, Oh GW, Lee EH, Son BS, Kim TJ (2017). "Functional expression and enzymatic characterization of cyclomaltodextrinase from Streptococcus pyogenes". Korean Journal of Microbiology. 53 (3): 208–215. doi:10.7845/kjm.2017.7062. ISSN 0440-2413.
- Baek JS, Kim HY, Abbott TP, Moon TW, Lee SB, Park CS, Park KH (March 2003). "Acarviosine-simmondsin, a novel compound obtained from acarviosine-glucose and simmondsin by Thermus maltogenic amylase and its in vivo effect on food intake and hyperglycemia". Bioscience, Biotechnology, and Biochemistry. 67 (3): 532–539. doi:10.1271/bbb.67.532. PMID 12723600. S2CID 2813481.
- Balaich J, Estrella M, Wu G, Jeffrey PD, Biswas A, Zhao L, et al. (December 2021). "The human microbiome encodes resistance to the antidiabetic drug acarbose". Nature. 600 (7887): 110–115. Bibcode:2021Natur.600..110B. doi:10.1038/s41586-021-04091-0. PMID 34819672. S2CID 244644880.
- Park, Kwan-Hwa (2006). "Function and Tertiary- and Quaternary-structure of Cyclodextrin-hydrolyzing Enzymes (CDase), a Group of Multisubstrate Specific Enzymes Belonging to the α-Amylase Family". Journal of Applied Glycoscience. 53 (1): 35–44. doi:10.5458/jag.53.35. ISSN 1344-7882.
- Bozonnet, S; Jensen, MT; Nielsen, MM; Aghajari, N; Jensen, MH; Kramhøft, B; Willemoës, M; Tranier, S; Haser, R; Svensson, B (October 2007). "The "pair of sugar tongs" site on the non-catalytic domain C of barley alpha-amylase participates in substrate binding and activity". The FEBS Journal. 274 (19): 5055–67. doi:10.1111/j.1742-4658.2007.06024.x. PMID 17803687. S2CID 25592455.
- Miyazaki, T; Park, EY (26 June 2020). "Structure-function analysis of silkworm sucrose hydrolase uncovers the mechanism of substrate specificity in GH13 subfamily 17 exo-α-glucosidases". The Journal of Biological Chemistry. 295 (26): 8784–8797. doi:10.1074/jbc.RA120.013595. PMC 7324511. PMID 32381508.
External links
- "Acarbose". Drug Information Portal. U.S. National Library of Medicine.
- "Probing the Pancreas" - by Craig D. Reid, Ph.D. (US FDA Consumer Article)