Tirzepatide
Tirzepatide, sold under the brand name Mounjaro, is a medication used for the treatment of type 2 diabetes.[1][2][3][4] Tirzepatide is given by weekly subcutaneous injection (under the skin).[1][2] Common side effects may include nausea, vomiting, diarrhea, constipation, upper abdominal discomfort, and abdominal pain.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Mounjaro |
| Other names | LY3298176, GIP/GLP-1 RA |
| License data |
|
| Routes of administration | Subcutaneous |
| Drug class | Antidiabetic, GLP-1 receptor agonist |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | Albumin |
| Metabolism | Proteolytic cleavage, β-oxidation of fatty diacid section and amide hydrolysis |
| Elimination half-life | Five days |
| Excretion | Urine and faeces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C225H348N48O68 |
| Molar mass | 4813.527 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are hormones involved in blood sugar control.[2] After a person has eaten, these hormones are secreted by cells of the intestines and in turn cause the secretion of insulin. Tirzepatide is a first-in-class medication. It is a GIP-analogue that activates both the GLP-1 and GIP receptors, leading to improved blood sugar control.[2]
Medical uses
Tirzepatide is indicated to improve blood sugar control in adults with type 2 diabetes, as an addition to diet and exercise.[1][2]
Contraindications
Tirzepatide should not be used in people with a personal or family history of medullary thyroid cancer or in people with multiple endocrine neoplasia syndrome type 2.[2]
Adverse effects
Preclinical, phase I, and phase II clinical trials indicated that tirzepatide exhibits adverse effects similar to those of other established GLP-1 receptor agonists, such as dulaglutide. These effects occur largely within the gastrointestinal tract.[5] The most frequently observed are nausea, diarrhea and vomiting, which increased in incidence with the dosage amount (i.e. higher likelihood the higher the dose). The number of patients who discontinued taking tirzepatide also increased as dosage increased, with patients taking 15 mg having a 25% discontinuation rate vs 5.1% for 5 mg patients and 11.1% for dulaglutide.[6] To a slightly lesser extent, patients also reported reduced appetite.[5] Other side effects reported were dyspepsia, constipation, abdominal pain, dizziness and hypoglycaemia.[7][8]
Pharmacology
Tirzepatide is an analogue of gastric inhibitory polypeptide (GIP), a human hormone that stimulates the release of insulin from the pancreas. Tirzepatide is a linear polypeptide of 39 amino acids that has been chemically modified by lipidation to improve its uptake into cells and its stability to metabolism.[9] It completed phase III trials globally in 2021.[10][11]
Mechanism of action
Tirzepatide has a greater affinity to GIP receptors than to GLP-1 receptors, and this dual agonist behavior has been shown to produce greater reductions of hyperglycemia compared to a selective GLP-1 receptor agonist.[3] Signaling studies reported that tirzepatide mimics the actions of natural GIP at the GIP receptor.[12] However, at the GLP-1 receptor, tirzepatide shows bias towards cAMP (a messenger associated with regulation of glycogen, sugar and lipid metabolism) generation, rather than β-arrestin recruitment. This combination of preference towards GIP receptor and distinct signaling properties at GLP-1 suggest this biased agonism increases insulin secretion.[12] Tirzepatide has been reported to increase levels of adiponectin, an adipokine involved in the regulation of both glucose and lipid metabolism, with a maximum increase of 26% from baseline after 26 weeks, at the 10 mg dosage.[3]
Chemistry
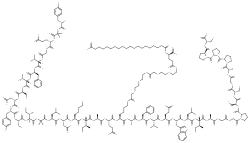
Structure
Tirzepatide is an analog of the human GIP hormone with a C20 fatty-diacid portion attached, used to optimise the uptake and metabolism of the compound.[9] The fatty-diacid section (eicosanedioic acid) is linked via a glutamic acid and two (2-(2-aminoethoxy)ethoxy)acetic acid units to the side chain of the lysine residue. This arrangement allows for a much longer half life, extending the time between doses, because of its high affinity to albumin.[13]
Synthesis
The synthesis of tirzepatide was first disclosed in patents filed by Eli Lilly and Company.[14] This uses standard solid phase peptide synthesis, with an allyloxycarbonyl protecting group on the lysine at position 20 of the linear chain of amino acids, allowing a final set of chemical transformations in which the sidechain amine of that lysine is derivatized with the lipid-containing fragment.
Large-scale manufacturing processes have been reported for this compound.[15]
History
Eli Lilly and Company first applied for a patent for a method of glycemic control using tirzepatide in early 2016.[14] The patent was published late that year. After passing phase III clinical trials, Lilly applied for FDA approval in October 2021, with a priority review voucher.[16]
Following the completion of the SURPASS-2 trial (NCT03987919), the company announced on 28 April 2022 that tirzepatide had successfully met their endpoints in obese and overweight patients without diabetes.[17]
In industry-funded preliminary trials comparing tirzepatide to the existing diabetes medication semaglutide (an injected analogue of the hormone GLP-1), tirzepatide showed minor improvement of reductions (2.01%–2.30% depending on dosage) in glycated hemoglobin tests relative to semaglutide (1.86%).[18] A 10 mg dose has also been shown to be effective in reducing insulin resistance, with a reduction of around 8% from baseline, measured using HOMA2-IR (computed with fasting insulin).[3] Fasting levels of IGF binding proteins like IGFBP1 and IGFBP2 increased following tirzepatide treatment, increasing insulin sensitivity.[3]
Meta-analysis
A 2021 meta-analysis showed that over one-year of clinical use, tirzepatide was observed to be superior to dulaglutide, semaglutide, degludec, and insulin glargine with regards to glycemic efficacy and obesity reduction.[19]
In a phase 3 double-blind, randomized, controlled trial supported by Eli Lilly, non-diabetic adults with a body mass index of 30 or more, or 27 or more and at least one weight-related complication, excluding diabetes, were randomized to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg). The mean percentage change in weight at week 72 was −15.0% (95% confidence interval [CI], −15.9 to −14.2) with 5-mg weekly doses of tirzepatide, −19.5% (95% CI, −20.4 to −18.5) with 10-mg doses, and −20.9% (95% CI, −21.8 to −19.9) with 15-mg doses and −3.1% (95% CI, −4.3 to −1.9) with placebo.[20][21][22]
Society and culture
Legal status
Tirzepatide was approved for medical use in the United States in May 2022.[1][2]
On 21 July 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Mounjaro, intended for the treatment of type 2 diabetes.[23]
Names
Tirzepatide is the international nonproprietary name (INN).[24]
References
- "Mounjaro- tirzepatide injection, solution". DailyMed. 13 May 2022. Archived from the original on 3 July 2022. Retrieved 27 May 2022.
- "FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes". U.S. Food and Drug Administration (FDA) (Press release). 13 May 2022. Archived from the original on 13 May 2022. Retrieved 13 May 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, et al. (January 2021). "Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes". The Journal of Clinical Endocrinology and Metabolism. 106 (2): 388–396. doi:10.1210/clinem/dgaa863. PMC 7823251. PMID 33236115.
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, et al. (December 2018). "LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept". Molecular Metabolism. 18: 3–14. doi:10.1016/j.molmet.2018.09.009. PMC 6308032. PMID 30473097.
- Min T, Bain SC (January 2021). "The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials". Diabetes Therapy. 12 (1): 143–157. doi:10.1007/s13300-020-00981-0. PMC 7843845. PMID 33325008.
- Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. (November 2018). "Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial". The Lancet. 392 (10160): 2180–2193. doi:10.1016/S0140-6736(18)32260-8. PMID 30293770.
- Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, et al. (June 2020). "Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens". Diabetes, Obesity & Metabolism. 22 (6): 938–946. doi:10.1111/dom.13979. PMC 7318331. PMID 31984598.
- Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á (February 2022). "Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial". JAMA. 327 (6): 534–545. doi:10.1001/jama.2022.0078. PMC 8826179. PMID 35133415.
- Ahangarpour M, Kavianinia I, Harris PW, Brimble MA (January 2021). "Photo-induced radical thiol-ene chemistry: a versatile toolbox for peptide-based drug design". Chemical Society Reviews. Royal Society of Chemistry. 50 (2): 898–944. doi:10.1039/d0cs00354a. PMID 33404559. S2CID 230783854.
- "Tirzepatide significantly reduced A1C and body weight in people with type 2 diabetes in two phase 3 trials from Lilly's SURPASS program" (Press release). Eli Lilly and Company. 17 February 2021. Archived from the original on 28 October 2021. Retrieved 28 October 2021 – via PR Newswire.
- "Lilly : Phase 3 Tirzepatide Results Show Superior A1C And Body Weight Reductions In Type 2 Diabetes". Business Insider. RTTNews. 19 October 2021. Archived from the original on 28 October 2021. Retrieved 28 October 2021.
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, et al. (September 2020). "Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist". JCI Insight. 5 (17). doi:10.1172/jci.insight.140532. PMC 7526454. PMID 32730231.
- Østergaard S, Paulsson JF, Kofoed J, Zosel F, Olsen J, Jeppesen CB, et al. (October 2021). "The effect of fatty diacid acylation of human PYY3-36 on Y2 receptor potency and half-life in minipigs". Scientific Reports. 11 (1): 21179. Bibcode:2021NatSR..1121179O. doi:10.1038/s41598-021-00654-3. PMC 8551270. PMID 34707178.
- US patent 9474780, Bokvist BK, Coskun T, Cummins RC, Alsina-Fernandez J, "GIP and GLP-1 co-agonist compounds", issued 2016-10-25, assigned to Eli Lilly and Co
- Frederick MO, Boyse RA, Braden TM, Calvin JR, Campbell BM, Changi SM, et al. (2021). "Kilogram-Scale GMP Manufacture of Tirzepatide Using a Hybrid SPPS/LPPS Approach with Continuous Manufacturing". Organic Process Research & Development. 25 (7): 1628–1636. doi:10.1021/acs.oprd.1c00108. S2CID 237690232.
- Sagonowsky, Eric (26 October 2021). "As Lilly gears up for key 2022 launches, Trulicity, Taltz and more drive solid growth". Fierce Pharma. Archived from the original on 14 May 2022. Retrieved 9 April 2022.
- Kellaher, Colin (28 April 2022). "Eli Lilly's Tirzepatide Meets Main Endpoints in Phase 3 Obesity Study". MarketWatch. Dow Jones Newswires. Archived from the original on 29 April 2022. Retrieved 29 April 2022.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. (August 2021). "Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes". The New England Journal of Medicine. 385 (6): 503–515. doi:10.1056/NEJMoa2107519. PMID 34170647. S2CID 235635529.
- Dutta D, Surana V, Singla R, Aggarwal S, Sharma M (November–December 2021). "Efficacy and safety of novel twincretin tirzepatide a dual GIP and GLP-1 receptor agonist in the management of type-2 diabetes: A Cochrane meta-analysis". Indian Journal of Endocrinology and Metabolism. 25 (6): 475–489. doi:10.4103/ijem.ijem_423_21. PMC 8959203. PMID 35355921.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. (4 June 2022). "Tirzepatide Once Weekly for the Treatment of Obesity". NEJM. 387 (3): 205–216. doi:10.1056/NEJMoa2206038. PMID 35658024. S2CID 249385412. Archived from the original on 5 June 2022. Retrieved 6 June 2022.
- Dee, Jane E. (6 June 2022). "More Than 20% Weight Reduction in Individuals With Obesity". Yale Department of Internal Medicine. Archived from the original on 10 June 2022. Retrieved 12 June 2022.
- Davis, Nicola (5 June 2022). "Diabetes drug leads to notable weight loss in people with obesity – study". The Guardian. Archived from the original on 11 June 2022. Retrieved 12 June 2022.
- "Mounjaro: Pending EC decision". European Medicines Agency. 22 July 2022. Archived from the original on 28 July 2022. Retrieved 30 July 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- World Health Organization (2019). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 81". WHO Drug Information. 33 (1). hdl:10665/330896.
Further reading
- Bhagavathula AS, Vidyasagar K, Tesfaye W (September 2021). "Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Phase II/III Trials". Pharmaceuticals (Basel). 14 (10): 991. doi:10.3390/ph14100991. PMC 8537322. PMID 34681215.
- Frías JP (November 2020). "Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes". Expert Rev Endocrinol Metab. 15 (6): 379–394. doi:10.1080/17446651.2020.1830759. PMID 33030356. S2CID 222213936.
- Ryan DH (September 2021). "Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice?". J Obes Metab Syndr. 30 (3): 196–208. doi:10.7570/jomes21033. PMC 8526285. PMID 34518444.
External links
- "Tirzepatide". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03954834 for "A Study of Tirzepatide (LY3298176) in Participants With Type 2 Diabetes Not Controlled With Diet and Exercise Alone (SURPASS-1)" at ClinicalTrials.gov
- Clinical trial number NCT03987919 for "A Study of Tirzepatide (LY3298176) Versus Semaglutide Once Weekly as Add-on Therapy to Metformin in Participants With Type 2 Diabetes (SURPASS-2)" at ClinicalTrials.gov
- Clinical trial number NCT03882970 for "A Study of Tirzepatide (LY3298176) Versus Insulin Degludec in Participants With Type 2 Diabetes (SURPASS-3)" at ClinicalTrials.gov
- Clinical trial number NCT03730662 for "A Study of Tirzepatide (LY3298176) Once a Week Versus Insulin Glargine Once a Day in Participants With Type 2 Diabetes and Increased Cardiovascular Risk (SURPASS-4)" at ClinicalTrials.gov
- Clinical trial number NCT04039503 for "A Study of Tirzepatide (LY3298176) Versus Placebo in Participants With Type 2 Diabetes Inadequately Controlled on Insulin Glargine With or Without Metformin (SURPASS-5)" at ClinicalTrials.gov