Semaglutide
Semaglutide, sold under the brand names Wegovy and Ozempic among others, is an antidiabetic medication used for the treatment of type 2 diabetes and long-term weight management.[16][17][18]
 | |
| Clinical data | |
|---|---|
| Trade names | Ozempic, Rybelsus, Wegovy, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618008 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Subcutaneous, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89% |
| Metabolism | Proteolysis |
| Elimination half-life | 7 days |
| Duration of action | 63.6 h |
| Excretion | Urine and feces |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.219.541 |
| Chemical and physical data | |
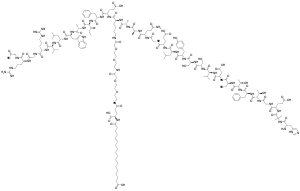
| Formula | C187H291N45O59 |
| Molar mass | 4113.641 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Semaglutide is a GLP-1 receptor agonist, meaning that it mimics the action of the human incretin glucagon-like peptide-1 (GLP-1), thereby increasing insulin secretion and increasing blood sugar disposal and improving glycemic control.
An injectable version (Ozempic) was approved for medical use in the United States in December 2017,[19] and in the European Union,[13] Canada,[20] and Japan in 2018. A version which is taken by mouth (Rybelsus) was approved for medical use in the United States in September 2019,[21] and in the European Union in April 2020.[14] It is the first glucagon-like peptide receptor protein treatment approved for use in the United States that does not need to be injected.[22] It was developed by Novo Nordisk. Side effects include nausea, vomiting, diarrhea, abdominal pain, and constipation.[10]
In June 2021, the US Food and Drug Administration (FDA) approved semaglutide injection sold under the brand name Wegovy for long-term weight management in adults.[12][18] It was approved for medical use in the European Union in January 2022.[15] In 2020, it was the 129th most commonly prescribed medication in the United States, with more than 4 million prescriptions.[23][24]
Medical uses
Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.[10][11]
Semaglutide is also indicated as an adjunct to diet and exercise for long-term weight management in adults with obesity (initial body mass index (BMI) ≥ 30 kg/m2) or overweight (initial BMI ≥ 27 kg/m2) with at least one weight-related comorbidity.[12][15][18] A 2022 review of anti-obesity treatments found that semaglutide as well as tirzepatide (which has an overlapping mechanism of action) were more promising than previous anti-obesity drugs, although less effective than bariatric surgery.[25]
Adverse effects
Side effects include nausea, vomiting, diarrhea, abdominal pain, and constipation.[10] In people with heart problems, it can cause damage to the retina of the eye (retinopathy).[26] Other, less common side effects include kidney problems, allergic reactions, low blood sugar, and pancreatitis.[22]
Contraindications
Due to data from rodents studies of GLP1 mediated thyroid C-cell hyperplasia,[27] their use is contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients with multiple endocrine neoplasia syndrome type 2.[11][10]
Mechanism of action
Semaglutide is a glucagon-like peptide-1 receptor agonist. By mimicking the action of the incretin GLP-1, it increases the production of insulin, a hormone that lowers the blood sugar level.[28] It appears to enhance growth of pancreatic beta cells, which are responsible for insulin production and release.[29][30] It also inhibits the production of glucagon, which is a hormone that increases glycogenolysis (release of stored carbohydrate from the liver) and gluconeogenesis (synthesis of new glucose). It reduces food intake by lowering appetite and slowing down digestion in the stomach,[26] helping to reduce body fat.[31] Its half-life in the blood is about seven days (165–184 hours).[29][32]
Structure
Semaglutide is chemically similar to human GLP-1, with 94% similarity. The only differences are two amino-acid substitutions at positions 8 and 34, where alanine and lysine are replaced by 2-aminoisobutyric acid and arginine, respectively.[33] Amino-acid substitution at position 8 prevents chemical breakdown by dipeptidyl peptidase-4. In addition, the lysine at position 26 is in its derivative form (acylated with stearic diacid). Acylation with a spacer and C-18 fatty diacid chain increases the drug's binding to blood protein (albumin), which enables longer presence in the blood circulation.[34]
History
Semaglutide was developed in 2012,[35] by a team of researchers at Novo Nordisk as a longer-acting alternative to liraglutide as a once-weekly diabetes therapy.[36] It was given the brand name Ozempic. Clinical trials were started on 6 January 2016, and completed on 19 May 2017.[37]
Researchers at the University of Leeds and Novo Nordisk reported in 2017, that it can also be used for the treatment of obesity.[38] It reduces hunger, food craving and body fat.[39] A phase 3 randomized controlled trial found that once-weekly injection of 2.4 mg of the drug resulted in an average change of −14.9% body weight at 68 weeks compared to −2.4% for the placebo.[40]
The US FDA New Drug Application (NDA) was filed in December 2016, and in October 2017, the FDA Advisory Committee approved it unanimously.[41] It can be administered by injection or orally.[42] Authorization was granted in February 2018 for the European Union,[13][43] in March 2018 in Japan,[44] on 4 January 2018 in Canada,[20] and in August 2019 in Australia.[1][3]
In November 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion on Wegovy, the formulation intended for the treatment of people with obesity or who are overweight in the presence of other related conditions, recommending the granting of a marketing authorization.[45] The applicant for this medicinal product is Novo Nordisk A/S.[45] Wegovy was approved for medical use in the European Union in January 2022.[15]
2022 shortages
As a result of an increase in prescriptions for weight loss in non-diabetic patients, a shortage of Semaglutide/Ozempic was reported in Australia in 2022.[46] This shortage resulted in many people with type 2 diabetes being unable to access the drug. In May 2022, the Australian Therapeutic Goods Administration, along with Novo Nordisk, sent out guidelines to all health professionals, reminding them that people with type 2 diabetes need to be prioritised in terms of supply. An update to the guidelines was released in August 2022.[47]
Shortages were also reported contemporaneously in the United States[48] In the same month, rival Eli Lilly stated in its quarterly investors call that it was working "around the clock" to ensure adequate supply of its rival GLP-1 agonist tirzepatide (Mounjaro).[49]
Research
Semaglutide was found to be inferior to tirzepatide in a 2021 study of tirzepatide (LY3298176) vs semaglutide once weekly as add-on therapy to metformin in participants with type 2 diabetes (SURPASS-2), in both endpoints of reduction in A1C and body weight, with a roughly similar safety profile.[50][51]
A meta-analysis including a small number of patients found that semaglutide may be effective in lowering liver enzymes (transaminitis) and improving certain radiologically observed[52] features of metabolic-dysfunction–associated fatty-liver disease (MAFLD).[53]
References
- "AusPAR: Semaglutide". Therapeutic Goods Administration (TGA). 2 December 2020. Archived from the original on 24 February 2022. Retrieved 23 February 2022.
- "Rybelsus APMDS". Therapeutic Goods Administration (TGA). 22 February 2022. Retrieved 23 February 2022.
- "Summary for ARTG Entry:315107 Ozempic 1 mg semaglutide (rys) 1.34 mg/mL solution for injection pre-filled pen". Therapeutic Goods Administration (TGA). Archived from the original on 24 February 2022. Retrieved 6 June 2021.
- "Summary for ARTG Entry: 346198 Rybelsus semaglutide 3 mg tablet blister pack". Therapeutic Goods Administration (TGA). Archived from the original on 24 February 2022. Retrieved 23 February 2022.
- Product Monograph Including Patient Medication Information – Ozempic semaglutide injection (PDF) (Report). Novo-Nordisk Canada. 21 August 2020 [Initial approval 4 January 2018]. Archived (PDF) from the original on 7 June 2021. Retrieved 6 June 2021.
- Product Monograph Including Patient Medication Information – Rybelsus semaglutide tablets (PDF) (Report). Novo-Nordisk Canada. 30 March 2020. Archived (PDF) from the original on 14 December 2021. Retrieved 6 June 2021.
- "Regulatory Decision Summary – Rybelsus". Health Canada. 23 October 2014. Archived from the original on 5 June 2022. Retrieved 4 June 2022.
- "Ozempic 0.25 mg solution for injection in pre-filled pen – Summary of Product Characteristics (SmPC)". (emc). 9 April 2021. Archived from the original on 6 June 2021. Retrieved 6 June 2021.
- "Rybelsus – Summary of Product Characteristics (SmPC)". (emc). 25 November 2020. Archived from the original on 6 June 2021. Retrieved 6 June 2021.
- "Ozempic- semaglutide injection, solution". DailyMed. Archived from the original on 5 June 2021. Retrieved 5 June 2021.
- "Rybelsus- oral semaglutide tablet". DailyMed. Archived from the original on 5 June 2021. Retrieved 5 June 2021.
- "Wegovy- semaglutide injection, solution". DailyMed. 4 June 2021. Archived from the original on 14 December 2021. Retrieved 11 March 2022.
- "Ozempic EPAR". European Medicines Agency (EMA). Archived from the original on 25 October 2020. Retrieved 26 September 2020.
- "Rybelsus EPAR". European Medicines Agency (EMA). 29 January 2020. Archived from the original on 14 August 2020. Retrieved 26 September 2020.
- "Wegovy EPAR". European Medicines Agency (EMA). 11 November 2021. Archived from the original on 2 July 2022. Retrieved 11 March 2022.
- "Semaglutide Approval Status". drugs.com. Archived from the original on 24 October 2017. Retrieved 18 May 2017.
- "Ozempic (semaglutide) approved in the US". Novo Nordisk (Press release). 5 December 2017. Archived from the original on 5 June 2021. Retrieved 5 June 2021.
- "FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014". U.S. Food and Drug Administration (FDA) (Press release). 4 June 2021. Archived from the original on 4 June 2021. Retrieved 5 June 2021.
- "Drug Approval Package: Ozempic (semaglutide) Injection". U.S. Food and Drug Administration (FDA). 16 January 2018. Archived from the original on 1 March 2021. Retrieved 26 September 2020.
- "Regulatory Decision Summary – Ozempic". Health Canada. 23 October 2014. Archived from the original on 17 May 2019. Retrieved 2 April 2019.
- "Drug Approval Package: Rybelsus". U.S. Food and Drug Administration (FDA). 10 June 2020. Archived from the original on 2 November 2020. Retrieved 26 September 2020.
- "FDA approves first oral GLP-1 treatment for type 2 diabetes" (Press release). FDA. 20 September 2019. Archived from the original on 23 September 2019. Retrieved 20 September 2019.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Semaglutide - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- Müller TD, Blüher M, Tschöp MH, DiMarchi RD (March 2022). "Anti-obesity drug discovery: advances and challenges". Nature Reviews. Drug Discovery. 21 (3): 201–223. doi:10.1038/s41573-021-00337-8. PMC 8609996. PMID 34815532.
- Doggrell SA (March 2018). "Semaglutide in type 2 diabetes - is it the best glucagon-like peptide 1 receptor agonist (GLP-1R agonist)?" (PDF). Expert Opinion on Drug Metabolism & Toxicology. 14 (3): 371–377. doi:10.1080/17425255.2018.1441286. PMID 29439603. S2CID 3421553. Archived (PDF) from the original on 5 May 2020. Retrieved 12 December 2019.
- Bjerre Knudsen, Lotte; Madsen, Lars Wichmann; Andersen, Søren; Almholt, Kasper; de Boer, Anne S.; Drucker, Daniel J.; Gotfredsen, Carsten; Egerod, Frederikke Lihme; Hegelund, Anne Charlotte; Jacobsen, Helene; Jacobsen, Søren Dyring; Moses, Alan C.; Mølck, Anne-Marie; Nielsen, Henriette S.; Nowak, Jette (4 March 2010). "Glucagon-Like Peptide-1 Receptor Agonists Activate Rodent Thyroid C-Cells Causing Calcitonin Release and C-Cell Proliferation". Endocrinology. 151 (4): 1473–1486. doi:10.1210/en.2009-1272. ISSN 0013-7227. PMID 20203154. S2CID 20934882. Archived from the original on 30 September 2022. Retrieved 19 September 2022.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. (November 2016). "Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes". The New England Journal of Medicine. 375 (19): 1834–1844. doi:10.1056/NEJMoa1607141. PMID 27633186. Archived from the original on 13 February 2021. Retrieved 5 September 2020.
- Goldenberg RM, Steen O (March 2019). "Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes". Canadian Journal of Diabetes. 43 (2): 136–145. doi:10.1016/j.jcjd.2018.05.008. PMID 30195966.
- Li Y, Hansotia T, Yusta B, Ris F, Halban P, Drucker D (2003). "Glucagon-like Peptide-1 Receptor Signaling Modulates β Cell Apoptosis". The Journal of Biological Chemistry. 278 (1): 471–478. doi:10.1074/jbc.M209423200. PMID 12409292.
- Dhillon S (February 2018). "Semaglutide: First Global Approval". Drugs. 78 (2): 275–284. doi:10.1007/s40265-018-0871-0. PMID 29363040. S2CID 46851453.
- Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A (May 2015). "Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel". Journal of Clinical Pharmacology. 55 (5): 497–504. doi:10.1002/jcph.443. PMC 4418331. PMID 25475122.
- Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, et al. (September 2015). "Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide". Journal of Medicinal Chemistry. 58 (18): 7370–80. doi:10.1021/acs.jmedchem.5b00726. PMID 26308095. S2CID 20228358.
- Gotfredsen CF, Mølck AM, Thorup I, Nyborg NC, Salanti Z, Knudsen LB, Larsen MO (July 2014). "The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates" (PDF). Diabetes. 63 (7): 2486–97. doi:10.2337/db13-1087. PMID 24608440. S2CID 35102048. Archived (PDF) from the original on 21 September 2017. Retrieved 22 August 2018.
- "Abstracts of the 48th EASD (European Association for the Study of Diabetes) Annual Meeting of the European Association for the Study of Diabetes. October 1-5, 2012. Berlin, Germany". Diabetologia. 55 (S1): S7-537. October 2012. doi:10.1007/s00125-012-2688-9. PMID 22918257.
- Kalra S, Gupta Y (July 2015). "Once-weekly glucagon-like peptide 1 receptor agonists". The Journal of the Pakistan Medical Association. 65 (7): 796–8. PMID 26160096. Archived from the original on 30 September 2022. Retrieved 10 April 2022.
- Clinical trial number NCT02648204 for "Efficacy and Safety of Semaglutide Versus Dulaglutide as add-on to Metformin in Subjects With Type 2 Diabetes" at ClinicalTrials.gov
- Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, Hjerpsted JB (September 2017). "Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity". Diabetes, Obesity & Metabolism. 19 (9): 1242–1251. doi:10.1111/dom.12932. PMC 5573908. PMID 28266779.
- "Drug can dramatically reduce weight of people with obesity". ScienceDaily. 23 October 2017. Archived from the original on 15 July 2019. Retrieved 24 October 2017.
- Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. (March 2021). "Once-Weekly Semaglutide in Adults with Overweight or Obesity". New England Journal of Medicine. 384 (11): 989–1002. doi:10.1056/NEJMoa2032183. ISSN 0028-4793. PMID 33567185. S2CID 231883214.
- "Development Status and FDA Approval Process for semaglutide". Drugs.com. 2017. Archived from the original on 24 October 2017. Retrieved 24 October 2017.
- Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OK, Jabbour S, Rosenstock J (October 2017). "Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial". JAMA. 318 (15): 1460–1470. doi:10.1001/jama.2017.14752. PMC 5817971. PMID 29049653.
- "Novo Nordisk A/S: Ozempic (semaglutide) approved in the EU for the treatment of type 2 diabetes" (Press release). Novo Nordisk A/S. 9 February 2018. Archived from the original on 2 April 2019. Retrieved 19 August 2018 – via GlobeNewswire.
- "Ozempic approved in Japan for the treatment of type 2 diabetes" (Press release). Novo Nordisk A/S. 23 March 2018. Archived from the original on 2 April 2019. Retrieved 2 April 2019 – via GlobeNewswire.
- "Wegovy : Pending EC decision". European Medicines Agency. 11 November 2021. Archived from the original on 13 November 2021. Retrieved 13 November 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Liotta M (27 May 2022). "Semaglutide shortage prompts caution around off-label prescribing". news GP. Archived from the original on 1 September 2022. Retrieved 1 September 2022.
- "Joint statement: Prioritisation of semaglutide (Ozempic) supply for people with type 2 diabetes during shortage". Therapeutic Goods Administration (TGA). Commonwealth of Australia. Archived from the original on 1 September 2022. Retrieved 1 September 2022.
- Jensen, Leslie; Wheeler, Michelle. "Current Drug Shortages : Semaglutide Injection". American Society of Health System Pharmacists. Archived from the original on 11 September 2022. Retrieved 11 September 2022.
- Stanton, Dan (12 August 2022). "Lilly working 'around the clock' to ensure Mounjaro supply". Bioprocess Insider. Archived from the original on 9 September 2022. Retrieved 11 September 2022.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. (August 2021). "Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes". The New England Journal of Medicine. 385 (6): 503–515. doi:10.1056/NEJMoa2107519. PMID 34170647. S2CID 235635529.
- "'Stunning' Twincretin Beats Semaglutide in Type 2 Diabetes". Medscape Medical News > Conference News > ADA 2021. 27 June 2021. Archived from the original on 3 July 2021. Retrieved 30 June 2021.
- Lee SS, Park SH (June 2014). "Radiologic evaluation of nonalcoholic fatty liver disease". World Journal of Gastroenterology. Baishideng Publishing Group Inc. 20 (23): 7392–7402. doi:10.3748/wjg.v20.i23.7392. PMC 4064084. PMID 24966609.
- Dutta D, Kumar M, Shivaprasad KS, Kumar A, Sharma M (June 2022). "Impact of semaglutide on biochemical and radiologic measures of metabolic-dysfunction associated fatty liver disease across the spectrum of glycaemia: A meta-analysis". Diabetes & Metabolic Syndrome. 16 (6): 102539. doi:10.1016/j.dsx.2022.102539. PMID 35709586. S2CID 249584781.
External links
- "Semaglutide". Drug Information Portal. U.S. National Library of Medicine.
- "Semaglutide". MedlinePlus.