Apixaban
Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa.[5][6][7] Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots.[5][7] It is used as an alternative to warfarin and does not require monitoring by blood tests[5] or dietary restrictions.[8] It is taken by mouth.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eliquis, others |
| Other names | BMS-562247-01 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613032 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~50% |
| Protein binding | ~87% |
| Metabolism | CYP3A4, CYP3A5, CYP1A2 and others |
| Elimination half-life | 9–14 h |
| Excretion | Bile duct (75%), kidney (25%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.332 |
| Chemical and physical data | |
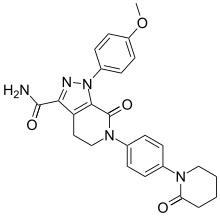
| Formula | C25H25N5O4 |
| Molar mass | 459.506 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Common side effects include bleeding and nausea.[5][6] Other side effects may include bleeding around the spine and allergic reactions.[5] Use is not recommended during pregnancy or breastfeeding.[1][6] Use appears to be relatively safe in those with mild kidney problems.[6] Compared to warfarin it has fewer interactions with other medications.[9] It is a direct factor Xa inhibitor.[5]
In 2007, Pfizer and Bristol-Myers Squibb began development of apixaban as an anticoagulant.[10] Apixaban was approved for medical use in the European Union in May 2011, and in the United States in December 2012.[4][5][11] It is on the World Health Organization's List of Essential Medicines.[12] In 2020, it was the 48th most commonly prescribed medication in the United States with more than 13 million prescriptions.[13][14] It is available as a generic medication.[15][16]
Medical uses
Apixaban is indicated for the following:[3]
- To lower the risk of stroke and embolism in people with nonvalvular atrial fibrillation.
- Deep vein thrombosis (DVT) prevention. DVTs may lead to pulmonary embolism (PE) in knee or hip replacement surgery patients.
- Treatment of both DVT and PE.
- To reduce the risk of recurring DVT and PE after initial therapy.
In the EU, apixaban is indicated for the prevention of venous thromboembolic events (VTE) in adults who have undergone elective hip or knee replacement surgery, the prevention of stroke and systemic embolism in adults with non-valvular atrial fibrillation (NVAF) with one or more risk factors, for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) in adults, and for the prevention of recurrent DVT and PE in adults.[4]
Atrial fibrillation
Apixaban is recommended by the National Institute for Health and Clinical Excellence for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation and at least one of the following risk factors: prior stroke or transient ischemic attack, age 75 years or older, diabetes mellitus, or symptomatic heart failure.[17]
Apixaban and other anticoagulants (dabigatran, edoxaban and rivaroxaban) appear equally effective as warfarin in preventing non-hemorrhagic stroke in people with atrial fibrillation and are associated with lower risk of intracranial bleeding.[18][19]
While apixaban may be used in people with severely decreased kidney function and those on hemodialysis it has not been studied in these groups.[5] Full dose apixaban (5 mg bid) can be used, unless at least two of the following characteristics apply: patient age is 80 years or older, body weight is 60 kg or less, and serum creatinine is 1.5 mg/dL or higher, in which case dose reduction to 2.5 mg bid is indicated.[20]
Side effects
Bleeding
Apixaban can increase the risk of bleeding which may be serious and potentially fatal. Concurrent use with other medications that affect blood clotting can further increase this risk. This includes medications such as other anticoagulants, heparin, aspirin, antiplatelet medications, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs).[3][21][22][23]
Andexanet alfa is a Food and Drug Administration (FDA) approved antidote for apixaban in people with uncontrolled and life-threatening bleeding events.[24][25]
Spinal puncture
Following spinal anesthesia or puncture, people who are being treated with anti-thrombotic agents are at higher risk for developing a hematoma, which can cause long-term or permanent paralysis. The risk of this may be increased by using epidural or intrathecal catheters after a surgical operation or from the concurrent use of medicinal agents that affect hemostasis.[3]
Mechanism of action
Apixaban is a highly selective, orally bioavailable, and reversible direct inhibitor of free and clot-bound factor Xa. Factor Xa catalyzes the conversion of prothrombin to thrombin, the final enzyme in the coagulation cascade that is responsible for fibrin clot formation.[26] Apixaban has no direct effect on platelet aggregation, but by inhibiting factor Xa, it indirectly decreases clot formation induced by thrombin.[3]
History
Apixaban was approved for medical use in the European Union in May 2011.[4]
A new drug application (NDA) for the approval of apixaban was submitted to the U.S. Food and Drug Administration (FDA) by Bristol-Myers Squibb (BMS) and Pfizer jointly after conclusion of the ARISTOTLE clinical trial in 2011.[27][11] Apixaban was approved for the prevention of stroke in people with atrial fibrillation on 28 December 2012.[11][28] On 13 March 2014, it was approved for the additional indication of preventing deep vein thrombosis and pulmonary embolism in people who have recently undergone knee or hip replacement.[29][30] On 21 August 2014, the FDA approved apixaban for the additional indication of the treatment of recurring deep vein thrombosis and pulmonary embolism.[29][31] During its development the drug was known as BMS-562247-01.[32] By late 2019, sales of the product by BMS accounted for thirty-percent of their quarterly revenue.[33]
In December 2019, the US FDA approved a generic version of apixaban produced jointly by Mylan and Micro Labs.[33][34] BMS and Pfizer worked quickly to block generics from being created, and in August 2020, they won a patent infringement lawsuit against Sigmapharm, Sunshine Lake, and Unichem, after previously settling patent cases against 25 other companies.[35][36] In September 2021, a Federal Circuit Court upheld the ruling.[37] The result is that apixaban generics will most likely not be available in the United States until at least 2026, but possibly 2031.[38]
In mid-2022, the Canadian generic drug company, Apotex Inc., obtained approval for marketing of apixaban.[39]
Research
Use in the COVID-19 pandemic
The UK National Health Service (NHS) is testing the use of apixaban in treating post-recovery COVID-19.[40]
References
- "Apixaban (Eliquis) Use During Pregnancy". Drugs.com. 21 June 2019. Retrieved 13 August 2020.
- "Eliquis 5 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 3 May 2022. Retrieved 8 October 2022.
- "Eliquis- apixaban tablet, film coated Eliquis 30-day starter pack- apixaban kit". DailyMed. 26 November 2019. Retrieved 22 April 2020.
- "Eliquis EPAR". European Medicines Agency. Retrieved 22 April 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Apixaban Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 27 March 2019.
- British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 124–125. ISBN 9780857113382.
- "FDA approves first generics of Eliquis". U.S. Food and Drug Administration (FDA). 23 December 2019. Archived from the original on 23 December 2019. Retrieved 23 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Hall, Harriet (September–October 2020). "How a Drug Is Born". Skeptical Inquirer. Amherst, New York: Center for Inquiry.
- Kiser, Kathryn (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438.
- "Bristol-Myers Squibb and Pfizer Announce Worldwide Collaboration to Develop and Commercialize Anticoagulant and Metabolic Compounds". Pfizer (Press release). Archived from the original on 10 September 2015. Retrieved 25 December 2021.
- "Drug Approval Package: Eliquis (apixaban) NDA #202155". U.S. Food and Drug Administration (FDA). 13 February 2013. Retrieved 23 December 2019.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Apixaban - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- "Apixaban 2.5mg Film-Coated Tablets - Summary of Product Characteristics (SmPC)". (emc). 27 July 2022. Retrieved 8 October 2022.
- "About apixaban". National Health Service. 16 May 2022. Retrieved 8 October 2022.
- "Apixaban for preventing stroke and systemic embolism in people with nonvalvular atrial fibrillation" (PDF). National Institute for Health and Care Excellence. January 2013. Retrieved 26 February 2016.
- Gómez-Outes, A; Terleira-Fernández, AI; Calvo-Rojas, G; Suárez-Gea, ML; Vargas-Castrillón, E (2013). "Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups". Thrombosis. 2013: 640723. doi:10.1155/2013/640723. PMC 3885278. PMID 24455237.
- Lowenstern, Angela; Al-Khatib, Sana M.; Sharan, Lauren; Chatterjee, Ranee; Allen LaPointe, Nancy M.; Shah, Bimal; Borre, Ethan D.; Raitz, Giselle; Goode, Adam; Yapa, Roshini; Davis, J. Kelly (4 December 2018). "Interventions for Preventing Thromboembolic Events in Patients With Atrial Fibrillation: A Systematic Review". Annals of Internal Medicine. 169 (11): 774–787. doi:10.7326/M18-1523. ISSN 0003-4819. PMC 6825839. PMID 30383133.
- However, the prescription information acknowledges that "clinical efficacy and safety studies with Eliquis did not enroll patients with end-stage renal disease (ESRD) on dialysis".
- "Atrial fibrillation and new oral anticoagulant drugs". U.S. Food and Drug Administration (FDA). 2 December 2015. Retrieved 22 April 2020.
- "Atrial fibrillation, oral anticoagulant drugs, and their reversal agents". U.S. Food and Drug Administration. 2 December 2015. Retrieved 22 April 2020.
- "No change is needed in use of direct oral anticoagulants following EMA-funded study". European Medicines Agency (EMA). 27 March 2020. Retrieved 22 April 2020.
- "Andexxa- andexanet alfa injection, powder, lyophilized, for solution". DailyMed. 8 January 2019. Retrieved 23 December 2019.
- "Andexxa (coagulation factor Xa (recombinant), inactivated-zhzo)". U.S. Food and Drug Administration (FDA). 31 December 2018. Retrieved 22 April 2020.
- Frost C, Wang J, Nepal S, et al. (February 2013). "Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects". Br J Clin Pharmacol. 75 (2): 476–87. doi:10.1111/j.1365-2125.2012.04369.x. PMC 3558798. PMID 22759198.
- Granger, Christopher (15 September 2011). "Apixaban versus Warfarin in Patients with Atrial Fibrillation". New England Journal of Medicine. 365 (11): 981–992. doi:10.1056/NEJMoa1107039. PMID 21870978. S2CID 43262809.
- Cada, Dennis J.; Levien, Terri L.; Baker, Danial E. (29 May 2013). "Apixaban". Hospital Pharmacy. 48 (6): 494–511. doi:10.1310/hpj4806-494. ISSN 0018-5787. PMC 3839491. PMID 24421512.
- "FDA-Approved Drugs: Eliquis (apixaban)". U.S. Food and Drug Administration (FDA). Retrieved 23 December 2019.
- Neale, Todd (14 March 2014). "FDA OKs Apixaban for DVT Prevention". MedPage Today. Retrieved 17 September 2015.
- "U.S. FDA Approves Eliquis (apixaban) for the Treatment of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), and for the Reduction in the Risk of Recurrent DVT and PE Following Initial Therapy" (Press release). Pfizer. 21 August 2014. Retrieved 26 February 2016.
- "Apixaban". PubChem, US National Library of Medicine. 27 August 2022. Retrieved 2 September 2022.
- "FIRST: Mylan, Micro Labs get USFDA nod for generic version of blood thinner Eliquis". Business Medical Dialogues. New Delhi, India: Minerva Medical Treatment. 24 December 2019. Retrieved 24 December 2019.
- Commissioner, Office of the (24 March 2020). "FDA approves first generics of Eliquis". U.S. Food and Drug Administration (FDA). Retrieved 30 November 2021.
- "Bristol-Myers Squibb Co. v. Aurobindo Pharma USA Inc". JD Supra. Retrieved 30 November 2021.
- "Bristol Myers, Pfizer fend off a key challenge to their top-selling heart drug". BioPharma Dive. Retrieved 30 November 2021.
- "Federal Circuit Crystallizes BMS' Apixaban District Court Win". The National Law Review. Retrieved 30 November 2021.
- "With Court Win, BMS and Pfizer Stave Off Generic Challengers to Eliquis – For Now". BioSpace. Retrieved 29 November 2021.
- Sandra Levy (1 August 2022). "Apotex offers generic Eliquis in Canada". Drugstore News. Retrieved 2 September 2022.
- "National drug trial to prevent deaths after Covid patients leave hospital". Cambridge University Hospitals. Retrieved 7 April 2021.
External links
- "Apixaban". Drug Information Portal. U.S. National Library of Medicine.