Avoparcin
Avoparcin is a glycopeptide antibiotic effective against Gram-positive bacteria. It has been used in agriculture as an additive to livestock feed to promote growth in chickens, pigs, and cattle.[1] It is also used as an aid in the prevention of necrotic enteritis in poultry.[1]
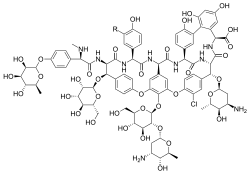 α-Avoparcin (R=H) β-Avoparcin (R=Cl) | |
| Identifiers | |
|---|---|
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.048.588 |
| E number | E715 (antibiotics) |
| KEGG |
|
PubChem CID |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C89H102ClN9O36 (α) C89H101Cl2N9O36 (β) |
| Molar mass | 1909.254 (α) 1943.699 (β) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Avoparcin is a mixture of two closely related chemical compounds, known as α-avoparcin and β-avoparcin, which differ by the presence of an additional chlorine atom in β-avoparcin. Avoparcin also shares a chemical similarity with vancomycin. Because of this similarity, concern exists that widespread use of avoparcin in animals may lead to an increased prevalence of vancomycin-resistant strains of bacteria.[2][3][4][5]
Avoparcin was once widely used in Australia and the European Union, but it is currently not permitted in either.[1][6] It was never approved for use in the United States.[7]
References
- "Avoparcin". Australian Pesticides and Veterinary Medicine Authority. Archived from the original on 2014-03-10. Retrieved 2012-09-19.
- Acar, J.; Casewell, M.; Freeman, J.; Friis, C.; Goossens, H. (2000). "Avoparcin and virginiamycin as animal growth promoters: A plea for science in decision-making". Clinical Microbiology and Infection. 6 (9): 477–82. doi:10.1046/j.1469-0691.2000.00128.x. PMID 11168181.
- Bager, F; Madsen, M; Christensen, J; Aarestrup, F.M (1997). "Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms". Preventive Veterinary Medicine. 31 (1–2): 95–112. doi:10.1016/S0167-5877(96)01119-1. PMID 9234429. S2CID 4958557.
- Peter J Collignon (1999). "Vancomycin-resistant enterococci and use of avoparcin in animal feed: is there a link?". Med J Aust. 171 (3): 144–146. doi:10.5694/j.1326-5377.1999.tb123568.x. PMID 10474607. S2CID 24378463.
- Lauderdale, TL; Shiau, YR; Wang, HY; Lai, JF; Huang, IW; Chen, PC; Chen, HY; Lai, SS; Liu, YF (2007). "Effect of banning vancomycin analogue avoparcin on vancomycin-resistant enterococci in chicken farms in Taiwan". Environmental Microbiology. 9 (3): 819–23. doi:10.1111/j.1462-2920.2006.01189.x. PMID 17298380.
- Commission Directive 97/6/EC of 30 January 1997 amending Council Directive 70/524/EEC concerning additives in feedingstuffs, Official Journal L 035 , 05/02/1997 P. 0011-0013
- Alex Koppelman (Nov 7, 2007). "Is the way we raise our food giving us MRSA?". Salon.com.