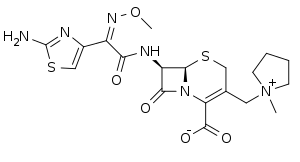
Cefepime
Cefepime is a fourth-generation cephalosporin antibiotic. Cefepime has an extended spectrum of activity against Gram-positive and Gram-negative bacteria, with greater activity against both types of organism than third-generation agents. A 2007 meta-analysis suggested when data of trials were combined, mortality was increased in people treated with cefepime compared with other β-lactam antibiotics.[1] In response, the U.S. Food and Drug Administration (FDA) performed their own meta-analysis which found no mortality difference.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈsɛfɪpiːm/ or /ˈkɛfɪpiːm/ |
| Trade names | Maxipime, Voco |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698021 |
| Pregnancy category |
|
| Routes of administration | Intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IM) |
| Metabolism | Hepatic 15% |
| Elimination half-life | 2 hours |
| Excretion | Renal 70–99% |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.171.025 |
| Chemical and physical data | |
| Formula | C19H24N6O5S2 |
| Molar mass | 480.56 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 150 °C (302 °F) (dec.) |
SMILES
| |
InChI
| |
| (verify) | |
Cefepime was patented in 1982 by Bristol-Myers Squibb and approved for medical use in 1994.[3] It is available as a generic drug and sold under a variety of trade names worldwide.
It was removed from the World Health Organization's List of Essential Medicines in 2019.[4]
Medical use
Cefepime is usually reserved to treat moderate to severe nosocomial pneumonia, infections caused by multiple drug-resistant microorganisms (e.g. Pseudomonas aeruginosa) and empirical treatment of febrile neutropenia.[5]
Cefepime has good activity against important pathogens including Pseudomonas aeruginosa, Staphylococcus aureus, and multiple drug-resistant Streptococcus pneumoniae. A particular strength is its activity against Enterobacteriaceae. Whereas other cephalosporins are degraded by many plasmid- and chromosome-mediated beta-lactamases, cefepime is stable and is a front-line agent when infection with Enterobacteriaceae is known or suspected.
Spectrum of bacterial susceptibility
Cefepime is a broad-spectrum cephalosporin antibiotic and has been used to treat bacteria responsible for causing pneumonia and infections of the skin and urinary tract. Some of these bacteria include Pseudomonas, Escherichia, and Streptococcus species. The following represents MIC susceptibility data for a few medically significant microorganisms:[6]
- Escherichia coli: ≤0.007 – 128 μg/ml
- Pseudomonas aeruginosa: 0.06 – >256 μg/ml
- Streptococcus pneumoniae: ≤0.007 – >8 μg/ml
Chemistry
The combination of the syn-configuration of the methoxy imino moiety and the aminothiazole moiety confers extra stability to β-lactamase enzymes produced by many bacteria. The N-methyl pyrrolidine moiety increases penetration into Gram-negative bacteria. These factors increase the activity of cefepime against otherwise resistant organisms including Pseudomonas aeruginosa and Staphylococcus aureus.
Trade names
Following expiration of the Bristol-Myers Squibb patent, cefepime became available as a generic and is now marketed by numerous companies worldwide under tradenames including Neopime (Neomed), Maxipime, Cepimax, Cepimex, and Axepim.
References
- Yahav D, Paul M, Fraser A, Sarid N, Leibovici L (2007). "Efficacy and safety of cefepime: a systematic review and meta-analysis". Lancet Infect Dis. 7 (5): 338–48. doi:10.1016/S1473-3099(07)70109-3. PMID 17448937.
- "Information for Healthcare Professionals: Cefepime (marketed as Maxipime)". Food and Drug Administration. Archived from the original on 2 November 2017. Retrieved 2 August 2009.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 496. ISBN 9783527607495. Archived from the original on 19 June 2021. Retrieved 19 September 2020.
- World Health Organization (2019). Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva: World Health Organization. hdl:10665/325773. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
- Chapman TM, Perry CM (2003). "Cefepime: a review of its use in the management of hospitalized patients with pneumonia". Am J Respir Med. 2 (1): 75–107. doi:10.1007/bf03256641. PMID 14720024.
- http://www.toku-e.com/Assets/MIC/Cefepime.pdf Archived 1 November 2018 at the Wayback Machine
External links
- "Cefepime". Drug Information Portal. U.S. National Library of Medicine.