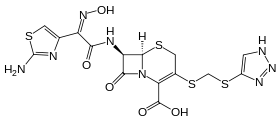
Cefmatilen
Cefmatilen (INN, codenamed S-1090) is an orally-active cephalosporin antibiotic. It was developed in Japan and first described in 1992.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H14N8O5S4 |
| Molar mass | 514.57 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
In vitro, cefmatilen is highly active against a variety of Gram-positive and Gram-negative bacteria, including Streptococcus pyogenes and Neisseria gonorrhoeae.[1]
References
- Tsuji M, Ishii Y, Ohno A, Miyazaki S, Yamaguchi K (November 1995). "In vitro and in vivo antibacterial activities of S-1090, a new oral cephalosporin". Antimicrob Agents Chemother. 39 (11): 2544–51. doi:10.1128/aac.39.11.2544. PMC 162981. PMID 8585742.
Further reading
- Cazzola M (February 2000). "Novel oral cephalosporins". Expert Opin Investig Drugs. 9 (2): 237–46. doi:10.1517/13543784.9.2.237. PMID 11060674. S2CID 45932120.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.