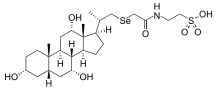
SeHCAT
SeHCAT (23-seleno-25-homotaurocholic acid, selenium homocholic acid taurine, or tauroselcholic acid) is a drug used in a clinical test to diagnose bile acid malabsorption.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(75Se)-2-[[[[(3α,5α,7α,12α,20S)-3,7,12-trihydroxy-20-methylpregnan-21-yl]seleno]acetyl]amino]ethanesulfonic acid, | |
| Other names
23-Seleno-25-homo-tauro-cholic acid; Selenium homocholic acid taurine; Tauroselcholic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C26H45NO7SSe |
| Molar mass | 594.68 g·mol−1 |
| Pharmacology | |
| V09DX01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Development
SeHCAT is a taurine-conjugated bile acid analog which was synthesized for use as a radiopharmaceutical to investigate in vivo the enterohepatic circulation of bile salts.[2] By incorporating the gamma-emitter 75Se into the SeHCAT molecule, the retention in the body or the loss of this compound into the feces could be studied easily using a standard gamma camera, available in most clinical nuclear medicine departments.
SeHCAT has been shown to be absorbed from the gut and excreted into the bile at the same rate as cholic acid, one of the major natural bile acids in humans. It undergoes secretion into the biliary tree, gallbladder and intestine in response to food, and is reabsorbed efficiently in the ileum, with kinetics similar to natural bile acids.[2][3] It was soon shown to be the most convenient and accurate method available to assess and measure bile acid turnover in the intestine.[4] SeHCAT testing was commercially developed by Amersham International Ltd (Amersham plc is now part of GE Healthcare Medical Diagnostics division) for clinical use to investigate malabsorption in patients with diarrhea. This test has replaced 14C-labeled glycocholic acid (or taurocholic acid) breath tests and fecal bile acid measurements, which now have no place in the routine clinical investigation of malabsorption.
Procedure
A capsule containing radiolabelled 75SeHCAT (with 370 kBq of Selenium-75 and less than 0.1 mg SeHCAT) is taken orally with water, to ensure passage of the capsule into the gastrointestinal tract. The physical half life of 75Se is approximately 118 days; activity is adjusted to a standard reference date.
Patients may be given instructions to fast prior to capsule administration; there is significant variation in clinical practice in this regard.[5] The effective dose of radiation for an adult given 370 kBq of SeHCAT is 0.26 mSv.[6] (For comparison, the radiation exposure from an abdominal CT scan is quoted at 5.3 mSv and annual background exposure in the UK 1-3 mSv.[7]) Measurements were originally performed with a whole-body counter but are usually performed now with an uncollimated gamma camera. The patient is scanned supine or prone with anterior and posterior acquisition from head to thigh 1 to 3 hours after taking the capsule. Scanning is repeated after 7 days. Background values are subtracted and care must be taken to avoid external sources of radiation in a nuclear medicine department.
From these measurements, the percent retention of SeHCAT at 7 days is calculated. A 7-day SeHCAT retention value greater than 15% is considered to be normal, with values less than 15% signifying excessive bile acid loss, as found in bile acid malabsorption.
With more frequent measurements, it is possible to calculate SeHCAT retention whole-body half-life; this is not routinely measured in a clinical setting. A half-life of greater than 2.8 days has been quoted as normal.[8]
Clinical use
The SeHCAT test is used to investigate patients with suspected bile acid malabsorption, who usually experience chronic diarrhea, often passing watery feces 5 to 10 times each day. When ileum has been removed following surgery, or is inflamed in Crohn's disease, the 7-day SeHCAT retention usually is abnormal, and most of these patients will benefit from treatment with bile acid sequestrants.[9][10] The enterohepatic circulation of bile acids is reduced in these patients with ileal abnormalities and, as the normal bile acid retention exceeds 95%, only a small degree of change is needed. Bile acid malabsorption can also be secondary to cholecystectomy, vagotomy and other disorders affecting intestinal motility or digestion such as radiation enteritis, celiac disease, and small intestinal bacterial overgrowth.
A similar picture of chronic diarrhea, an abnormal SeHCAT retention and a response to bile acid sequestrants, in the absence of other disorders of the intestine, is characteristic of idiopathic bile acid malabsorption – also called primary bile acid diarrhea. These patients are frequently misdiagnosed as having the irritable bowel syndrome, as clinicians fail to recognize the condition, do not think of performing a SeHCAT test, or do not have it available.[11]
There have been at least 18 studies of the use of SeHCAT testing in diarrhea-predominant irritable bowel syndrome patients. When these data were combined, 32% of 1223 patients had a SeHCAT 7-day retention of less than 10%, and 80% of these reported a response to cholestyramine, a bile acid sequestrant.[12]
References
- "23-seleno-25-homotaurocholic acid - Compound Summary". CID 123829. NCBI. Retrieved 3 April 2011.
- Boyd, GS; Merrick, MV; Monks, R; Thomas, IL (1981). "Se-75-labeled bile acid analogs, new radiopharmaceuticals for investigating the enterohepatic circulation". Journal of Nuclear Medicine. 22 (8): 720–5. PMID 7264761.
- Jazrawi, RP; Ferraris, R; Bridges, C; Northfield, TC (1988). "Kinetics for the synthetic bile acid 75selenohomocholic acid-taurine in humans: comparison with [14C]taurocholate". Gastroenterology. 95 (1): 164–9. PMID 3371611.
- Thaysen, EH; Orholm, M; Arnfred, T; Carl, J; Rødbro, P (October 1982). "Assessment of ileal function by abdominal counting of the retention of a gamma emitting bile acid analogue". Gut. 23 (10): 862–5. doi:10.1136/gut.23.10.862. PMC 1419815. PMID 7117906.
- Smith, MJ; Perkins, AC (2013). "A Survey of the clinical use of SeHCAT in the UK". Nuc. Med. Comms. 34 (4): 306–13. doi:10.1097/MNM.0b013e32835e8989. PMID 23407368.
- SeHCAT. SmPC 2008. GE Healthcare 1-7.
- Shrimpton PC, Hillier MC, Lewis MA, Dunn M (December 2006). "National survey of doses from CT in the UK: 2003". The British Journal of Radiology. 79 (948): 968–80. doi:10.1259/bjr/93277434. PMID 17213302.
- van Tilburg AJ, de Rooij FW, van den Berg JW, Kooij PP, van Blankenstein M (1991). "The selenium-75-homocholic acid taurine test reevaluated: combined measurement of fecal selenium-75 activity and 3 alpha-hydroxy bile acids in 211 patients". Journal of Nuclear Medicine. 32 (6): 1219–24. PMID 2045936.
- Nyhlin, H; Merrick, MV; Eastwood, MA (1994). "Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT". Gut. 35 (1): 90–3. doi:10.1136/gut.35.1.90. PMC 1374639. PMID 8307458.
- Smith, MJ; Cherian, P; Raju, GS; Dawson, BF; Mahon, S; Bardhan, KD (2000). "Bile acid malabsorption in persistent diarrhoea". Journal of the Royal College of Physicians of London. 34 (5): 448–51. PMID 11077656.
- Walters, JR (2010). "Defining primary bile acid diarrhea: making the diagnosis and recognizing the disorder". Expert Review of Gastroenterology & Hepatology. 4 (5): 561–7. doi:10.1586/egh.10.54. PMID 20932141.
- Wedlake, L; A'Hern, R; Russell, D; Thomas, K; Walters, JR; Andreyev, HJ (2009). "Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome". Alimentary Pharmacology & Therapeutics. 30 (7): 707–17. doi:10.1111/j.1365-2036.2009.04081.x. PMID 19570102.