Vasopressin
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˌveɪzoʊˈprɛsɪn/ |
| Trade names | Vasostrict, Reverpleg, Empressin, others |
| Other names | Arginine vasopressin; argipressin |
IUPAC name
| |
| Clinical data | |
| Main uses | Shock, cardiac arrest, diabetes insipidus, paralytic ileus, gastrointestinal bleeding[1] |
| Side effects | Stomach ache, world spinning, flatulence, headache, tremor[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Breastfeeding | No evidence of harm[3] |
| Routes of use | Intravenous (IV), intramuscular (IM), subcutaneous (SC)[1] |
| Onset of action | Rapid[1] |
| Duration of action | 20 min (after IV stopped)[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Physiological data | |
| Source tissues | Supraoptic nucleus; Paraventricular nucleus of hypothalamus |
| Target tissues | System-wide |
| Receptors | V1A, V1B, V2, OXTR |
| Agonists | Felypressin, desmopressin |
| Antagonists | Diuretics |
| Metabolism | Predominantly in the liver and kidneys |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 1% |
| Metabolism | Predominantly in the liver and kidneys |
| Elimination half-life | 10-20 minutes |
| Excretion | Urine |
| Chemical and physical data | |
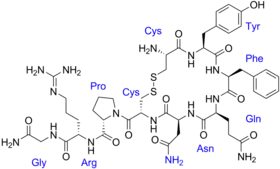
| Formula | C46H65N15O12S2 |
| Molar mass | 1084.24 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.6±0.1 g/cm3 |
SMILES
| |
InChI
| |
Vasopressin, sold under the brand name Argipressin among others, is a medication used for shock not responding to fluid resuscitation or norepinephrine.[1] Other uses include cardiac arrest, diabetes insipidus, paralytic ileus, and gastrointestinal bleeding.[1] It may also be used as part of a number of medical tests.[1] It is given by injection.[1] Onset of effects is rapid with the maximum effect at 15 min.[1]
Common side effects include stomach ache, world spinning, flatulence, headache, or tremor.[1] Other side effects may include a slow heart rate, mesenteric ischemia, water intoxication, and coronary ischemia.[4][1] During the later part of pregnancy it may result in contraction of the uterus.[5] It works by activating the vasopressin receptor resulting in contraction of blood vessels.[4] In the kidneys in results in concentration of the urine.[4]
Vasopressin was first isolated by Vincent du Vigneaud in 1953 and manufactured in 1958.[6] It is available as a generic medication.[3] In the United Kingdom a vial of 20 units costs the NHS about £80 as of 2020.[3] This amount in the United States costs about 60 USD.[7]
Medical uses
Vasopressin is used to manage anti-diuretic hormone deficiency. It has off-label uses and is used in the treatment of gastrointestinal bleeding, ventricular tachycardia and ventricular defibrillation. Vasopressin is used to treat diabetes insipidus related to low levels of antidiuretic hormone. It is available as Pressyn.[8]
Vasopressin agonists are used therapeutically in various conditions, and its long-acting synthetic analogue desmopressin is used in conditions featuring low vasopressin secretion, as well as for control of bleeding (in some forms of von Willebrand disease and in mild haemophilia A) and in extreme cases of bedwetting by children. Terlipressin and related analogues are used as vasoconstrictors in certain conditions. Use of vasopressin analogues for esophageal varices commenced in 1970.[9]
Vasopressin infusions are also used as second line therapy in septic shock patients not responding to fluid resuscitation or infusions of catecholamines (e.g., dopamine or norepinephrine).
Catecholamine refractory shock
Efficacy of vasopressin on systemic hemodynamics in catecholamine-resistant septic and postcardiotomy shock have been studied and published first in 2001[10] Later, the group concluded the ischemic skin lesions (ISL) developed in patients with catecholamine-resistant vasodilatory shock have multi-factorial cause and shall not necessarily been seen a side effect of AVP solely. The presence of septic shock and a history of peripheral arterial occlusive disease are independent risk factors for the development of ISL.[11] In the last decade, in early hyperdynamic septic shock, the administration of high-dose AVP as a single agent proved to fail to increase mean arterial pressure in the first hour but maintains it above 70mmHg in two-thirds of patients at 48h. AVP decreases NE exposure, has no effect on the PrCO(2) - PaCO(2 )difference, and improves renal function and SOFA score.[12] This led to development of a large trial to see theeffect of arginin vasopressin as add-on to norepinephrine in septic shock.[13] It could be shown, if giving vasopressin in early stage of septic shock (norepinephrin < 15 microgramm/min and lactate < 1.4 mmol/L) there is a statistically significant interaction between vasopressin and corticosteroids. The combination of low-dose vasopressin and corticosteroids was associated with decreased 28 and 90 days mortality and organ dysfunction compared with norepinephrine and corticosteroids.[14]
2018 Surviving Sepsis Campaign
The Surviving Sepsis Campaign guidelines recommend the very early management of the sepsis focusing on the hour-1 bundle. This includes use of Vasopressin 0.03 units/minute as add-on to norepinephrine (NE) with intent of either raising the mean arterial pressure or decreasing the norepinephrine dosage (i.e. de-catecholaminization).[15]
Cardiac arrest
Modern interest in vasopressors as a treatment for cardiac arrest stem mostly from canine studies performed in the 1960s by anesthesiologists Dr. John W. Pearson and Dr. Joseph Stafford Redding in which they demonstrated improved outcomes with the use of adjunct intracardiac epinephrine injection during resuscitation attempts after induced cardiac arrest.[16] Also contributing to the idea that vasopressors may be useful treatments in cardiac arrest are studies performed in the early to mid 1990s that found significantly higher levels of endogenous serum vasopressin in adults after successful resuscitation from out-of-hospital cardiac arrest compared to those who did not live.[17][18] Results of animal models have supported the use of either vasopressin or epinephrine in cardiac arrest resuscitation attempts, showing improved coronary perfusion pressure[19] and overall improvement in short-term survival as well as neurological outcomes.[20]
Vasopressin vs. epinephrine
Although both vasopressors, vasopressin and epinephrine differ in that vasopressin does not have direct effects on cardiac contractility as epinephrine does.[20] Thus, vasopressin is theorized to be of increased benefit over epinephrine in cardiac arrest due to its properties of not increasing myocardial and cerebral oxygen demands.[20] This idea has led to the advent of several studies searching for the presence of a clinical difference in benefit of these two treatment choices. Initial small studies demonstrated improved outcomes with vasopressin in comparison to epinephrine.[21] However, subsequent studies have not all been in agreement. Several randomized controlled trials have been unable to reproduce positive results with vasopressin treatment in both return of spontaneous circulation (ROSC) and survival to hospital discharge,[21][22][23][24] including a systematic review and meta-analysis completed in 2005 that found no evidence of a significant difference with vasopressin in five studied outcomes.[19]
Vasopressin and epinephrine vs. epinephrine alone
There is no current evidence of significant survival benefit with improved neurological outcomes in patients given combinations of both epinephrine and vasopressin during cardiac arrest.[19][22][25][26] A systematic review from 2008 did, however, find one study that showed a statistically significant improvement in ROSC and survival to hospital discharge with this combination treatment; unfortunately, those patients that survived to hospital discharge had overall poor outcomes and many suffered permanent, severe neurological damage.[24][26] A more recently published clinical trial out of Singapore has shown similar results, finding combination treatment to only improve the rate of survival to hospital admission, especially in the subgroup analysis of patients with longer "collapse to emergency department" arrival times of 15 to 45 minutes.[27]
Congenital heart disease
Vasopressin is used in managing hemodynamic instability in newborns and older children recovering from cardiac surgery.[28][29][30][31][32][33] There is evidence that some children recovering from cardiac surgery have relative vasopressin deficiency, such that their endogenous plasma concentrations of arginine vasopressin are lower than what would be expected in this clinical setting.[29][30][33] Though low endogenous vasopressin concentrations in and of themselves do not cause hemodynamic instability, neonates and children recovering from cardiac surgery who develop hemodynamic instability and have low endogenous vasopressin concentrations are optimal candidates for this surgery. Unfortunately, measurement of endogenous vasopressin concentration is time consuming and cumbersome, and not practical for bedside application. Copeptin, a more stable and easily measured product of pro-AVP processing, may be a means of identifying patients with low endogenous vasopressin concentrations.[30] Further research is needed. Also, systemic corticosteroids have been shown to suppress endogenous vasopressin production and release.[33] Neonates and children recovering from cardiac surgery who are receiving systemic corticosteroid therapy may also be optimal candidates for vasopressin therapy should hemodynamic instability be present.
Vasopressin receptor inhibition
A vasopressin receptor antagonist is an agent that interferes with action at the vasopressin receptors. They can be used in the treatment of hyponatremia.[34]
Dosage
For low blood pressure, generally 2.5 ml of 20 unit per mL solution is mixed in 500 mL of normal saline to give a solution of 0.1 units per mL.[1] This is than often started at 0.03 units per minute (1.8 units/hr).[35] Though a range of 0.015 to 0.1 units per minute (0.15 to 1 mL per min) may be used.[4][1] It is generally used as add on to norepinephrine when more than 15 ug/min is required.[35][36] In cardiac arrest a single dose of 40 units iv may be used.[4]
For diabetes insipidus 5 to 20 units either IM or SC every four hours may be used.[3]
Side effects
The most common side effects during treatment with vasopressin are dizziness, angina, chest pain, abdominal cramps, heartburn, nausea, vomiting, trembling, fever, water intoxication, pounding sensation in the head, diarrhoea, sweating, paleness, and flatulence. The most severe adverse reactions are myocardial infarction and hypersensitivity.[8]
Contraindications
The use of lysine vasopressin is contraindicated in the presence of hypersensitivity to beef or pork proteins, increased BUN and chronic kidney failure. It is recommended that it be cautiously used in instances of perioperative polyuria, sensitivity to the drug, asthma, seizures, heart failure, a comatose state, migraine headaches, and cardiovascular disease.[8]
Interactions
- alcohol - may lower the antidiuretic effect
- carbamazepine, chloropropamide, clofibrate, tricyclic antidepressants and fludrocortisone may raise the diuretic effect
- lithium, demeclocycline, heparin or norepinephrine may lower the antidiuretic effect
- vasopressor effect may be higher with the concurrent use of ganglionic blocking medications[8]
Pharmacokinetics
Vasopressin is administered through an intravenous device, intramuscular injection or a subcutaneous injection. The duration of action depends on the mode of administration and ranges from thirty minutes to two hours. It has a half life of ten to twenty minutes. It is widely distributed throughout the body and remains in the extracellular fluid. It is degraded by the liver and excreted through the kidneys.[8] Arginin vasopressins for use in septic shock are intended for intravenous use only.
History
Vasopressin was first isolated by Vincent du Vigneaud in 1953 and manufactured in 1958.[6]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Vasopressin Monograph for Professionals". Drugs.com. Archived from the original on 8 February 2018. Retrieved 15 April 2021.
- 1 2 "Vasopressin Use During Pregnancy". Drugs.com. 22 January 2020. Archived from the original on 26 November 2020. Retrieved 7 September 2020.
- 1 2 3 4 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 706. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 3 4 5 "VASOPRESSIN (PressynR) | LHSC". www.lhsc.on.ca. Archived from the original on 27 August 2021. Retrieved 16 April 2021.
- ↑ "Vasopressin Use During Pregnancy". Drugs.com. Archived from the original on 26 November 2020. Retrieved 16 April 2021.
- 1 2 Sneader, Walter (2006). Drug Discovery: A History. John Wiley & Sons. p. 168. ISBN 978-0-471-89979-2. Archived from the original on 2021-08-27. Retrieved 2021-04-15.
- ↑ "Vasopressin Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 12 November 2020. Retrieved 16 April 2021.
- 1 2 3 4 5 "Vasopressin" (PDF). F.A. Davis Company. 2017. Retrieved 2017-03-13.
{{cite web}}: CS1 maint: url-status (link) - ↑ Baum S, Nusbaum M, Tumen HJ (1970). "The control of gastrointestinal hemorrhage by selective mesenteric infusion of pitressin". Gastroenterology. 58: 926.
- ↑ Dünser MW, Mayr AJ, Ulmer H, Ritsch N, Knotzer H, Pajk W, et al. (July 2001). "The effects of vasopressin on systemic hemodynamics in catecholamine-resistant septic and postcardiotomy shock: a retrospective analysis". primary. Anesthesia and Analgesia. 93 (1): 7–13. doi:10.1097/00000539-200107000-00003. PMID 11429329. S2CID 41265162.
- ↑ Dünser MW, Mayr AJ, Tür A, Pajk W, Barbara F, Knotzer H, Ulmer H, Hasibeder WR (May 2003). "Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors". primary. Critical Care Medicine. 31 (5): 1394–8. doi:10.1097/01.CCM.0000059722.94182.79. PMID 12771608. S2CID 11514445.
- ↑ Lauzier F, Lévy B, Lamarre P, Lesur O (November 2006). "Vasopressin or norepinephrine in early hyperdynamic septic shock: a randomized clinical trial". primary. Intensive Care Medicine. 32 (11): 1782–9. doi:10.1007/s00134-006-0378-0. PMID 17019548. S2CID 25452934.
- ↑ Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D (February 2008). "Vasopressin versus norepinephrine infusion in patients with septic shock" (PDF). primary. The New England Journal of Medicine. 358 (9): 877–87. doi:10.1056/NEJMoa067373. PMID 18305265. Archived (PDF) from the original on 2021-08-27. Retrieved 2019-12-14.
- ↑ Russell JA, Walley KR, Gordon AC, Cooper DJ, Hébert PC, Singer J, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ (March 2009). "Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock". primary. Critical Care Medicine. 37 (3): 811–8. CiteSeerX 10.1.1.325.6984. doi:10.1097/CCM.0b013e3181961ace. PMID 19237882. S2CID 8267590.
- ↑ Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. (Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup) (February 2013). "Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012" (PDF). Critical Care Medicine. 41 (2): 580–637. doi:10.1097/CCM.0b013e31827e83af. PMID 23353941. S2CID 34855187. Archived from the original (PDF) on 2018-04-03. Retrieved 2018-09-11.
- ↑ Pearson JW, Redding JS (Sep–Oct 1963). "The Role of Epinephrine in Cardiac Resuscition". Anesthesia and Analgesia. 42 (5): 599–606. doi:10.1213/00000539-196309000-00022. PMID 14061643. S2CID 45444406.
- ↑ Lindner KH, Strohmenger HU, Ensinger H, Hetzel WD, Ahnefeld FW, Georgieff M (October 1992). "Stress hormone response during and after cardiopulmonary resuscitation". Anesthesiology. 77 (4): 662–8. doi:10.1097/00000542-199210000-00008. PMID 1329579.
- ↑ Lindner KH, Haak T, Keller A, Bothner U, Lurie KG (February 1996). "Release of endogenous vasopressors during and after cardiopulmonary resuscitation". Heart. 75 (2): 145–50. doi:10.1136/hrt.75.2.145. PMC 484250. PMID 8673752.
- 1 2 3 Aung K, Htay T (January 2005). "Vasopressin for cardiac arrest: a systematic review and meta-analysis". Archives of Internal Medicine. 165 (1): 17–24. doi:10.1001/archinte.165.1.17. PMID 15642869.
- 1 2 3 Williamson K, Breed M, Alibertis K, Brady WJ (February 2012). "The impact of the code drugs: cardioactive medications in cardiac arrest resuscitation". Emergency Medicine Clinics of North America. 30 (1): 65–75. doi:10.1016/j.emc.2011.09.008. PMID 22107975.
- 1 2 Lee SW (August 2011). "Drugs in resuscitation: an update". Singapore Medical Journal. 52 (8): 596–602. PMID 21879219.
- 1 2 Callaway CW, Hostler D, Doshi AA, Pinchalk M, Roth RN, Lubin J, Newman DH, Kelly LJ (November 2006). "Usefulness of vasopressin administered with epinephrine during out-of-hospital cardiac arrest". The American Journal of Cardiology. 98 (10): 1316–21. doi:10.1016/j.amjcard.2006.06.022. PMID 17134621.
- ↑ Stiell IG, Hébert PC, Wells GA, Vandemheen KL, Tang AS, Higginson LA, Dreyer JF, Clement C, Battram E, Watpool I, Mason S, Klassen T, Weitzman BN (July 2001). "Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial". Lancet. 358 (9276): 105–9. doi:10.1016/S0140-6736(01)05328-4. PMID 11463411. S2CID 31444934.
- 1 2 Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH (January 2004). "A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation". The New England Journal of Medicine. 350 (2): 105–13. doi:10.1056/NEJMoa025431. PMID 14711909. S2CID 33578302. Archived from the original on 2021-08-27. Retrieved 2019-12-14.
- ↑ Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien PY, Mauriaucourt P, Bragança C, Billères X, Clotteau-Lambert MP, Fuster P, Thiercelin D, Debaty G, Ricard-Hibon A, Roux P, Espesson C, Querellou E, Ducros L, Ecollan P, Halbout L, Savary D, Guillaumée F, Maupoint R, Capelle P, Bracq C, Dreyfus P, Nouguier P, Gache A, Meurisse C, Boulanger B, Lae C, Metzger J, Raphael V, Beruben A, Wenzel V, Guinhouya C, Vilhelm C, Marret E (July 2008). "Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation". The New England Journal of Medicine. 359 (1): 21–30. doi:10.1056/NEJMoa0706873. PMID 18596271.
- 1 2 Sillberg VA, Perry JJ, Stiell IG, Wells GA (December 2008). "Is the combination of vasopressin and epinephrine superior to repeated doses of epinephrine alone in the treatment of cardiac arrest-a systematic review". Resuscitation. 79 (3): 380–6. doi:10.1016/j.resuscitation.2008.07.020. PMID 18951676.
- ↑ Ong ME, Tiah L, Leong BS, Tan EC, Ong VY, Tan EA, Poh BY, Pek PP, Chen Y (August 2012). "A randomised, double-blind, multi-centre trial comparing vasopressin and adrenaline in patients with cardiac arrest presenting to or in the Emergency Department". Resuscitation. 83 (8): 953–60. doi:10.1016/j.resuscitation.2012.02.005. PMID 22353644.
- ↑ Mastropietro CW, Clark JA, Delius RE, Walters HL, Sarnaik AP (September 2008). "Arginine vasopressin to manage hypoxemic infants after stage I palliation of single ventricle lesions". Pediatric Critical Care Medicine. 9 (5): 506–10. doi:10.1097/pcc.0b013e3181849ce0. PMID 18679141. S2CID 30271090.
- 1 2 Mastropietro CW, Rossi NF, Clark JA, Chen H, Walters H, Delius R, Lieh-Lai M, Sarnaik AP (October 2010). "Relative deficiency of arginine vasopressin in children after cardiopulmonary bypass". Critical Care Medicine. 38 (10): 2052–8. doi:10.1097/ccm.0b013e3181eed91d. PMID 20683257. S2CID 46667831.
- 1 2 3 Mastropietro CW, Mahan M, Valentine KM, Clark JA, Hines PC, Walters HL, Delius RE, Sarnaik AP, Rossi NF (December 2012). "Copeptin as a marker of relative arginine vasopressin deficiency after pediatric cardiac surgery". Intensive Care Medicine. 38 (12): 2047–54. doi:10.1007/s00134-012-2731-9. PMID 23093248. S2CID 23426960.
- ↑ Mastropietro CW, Davalos MC, Seshadri S, Walters HL, Delius RE (June 2013). "Clinical response to arginine vasopressin therapy after paediatric cardiac surgery". Cardiology in the Young. 23 (3): 387–93. doi:10.1017/S1047951112000996. PMID 22805534.
- ↑ Davalos MC, Barrett R, Seshadri S, Walters HL, Delius RE, Zidan M, Mastropietro CW (March 2013). "Hyponatremia during arginine vasopressin therapy in children following cardiac surgery". Pediatric Critical Care Medicine. 14 (3): 290–7. doi:10.1097/pcc.0b013e3182720473. PMID 23392370. S2CID 35061631.
- 1 2 3 Mastropietro CW, Miletic K, Chen H, Rossi NF (December 2014). "Effect of corticosteroids on arginine vasopressin after pediatric cardiac surgery". Journal of Critical Care. 29 (6): 982–6. doi:10.1016/j.jcrc.2014.07.007. PMID 25092616.
- ↑ Palm C, Pistrosch F, Herbrig K, Gross P (July 2006). "Vasopressin antagonists as aquaretic agents for the treatment of hyponatremia". The American Journal of Medicine. 119 (7 Suppl 1): S87–92. doi:10.1016/j.amjmed.2006.05.014. PMID 16843091.
- 1 2 "UpToDate". www.uptodate.com. Archived from the original on 13 October 2022. Retrieved 25 May 2023.
- ↑ "BC Sepsis Network Guidelines" (PDF). Archived (PDF) from the original on 20 October 2023. Retrieved 13 October 2023.
External links
| Identifiers: |
|---|
- "Vasopressin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-21. Retrieved 2020-09-07.