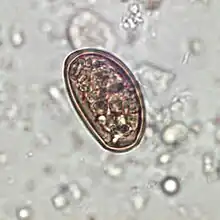
Dicrocoelium dendriticum
| Dicrocoelium dendriticum | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | |
| Order: | Plagiorchiida |
| Family: | Dicrocoeliidae |
| Genus: | Dicrocoelium |
| Species: | D. dendriticum |
| Binomial name | |
| Dicrocoelium dendriticum (Rudolphi, 1819) | |
Dicrocoelium dendriticum, the lancet liver fluke, is a parasite fluke that tends to live in cattle or other grazing mammals.[1]
Morphology
Dicrocoelium dendriticum has a similar morphology to Clonorchis sinensis, the Chinese liver fluke. Dicrocoelium dendriticum is distinguished by lobed testes in the anterior of the body, as opposed to Clonorchis sinensis whose testes are located in the posterior. They both are flat and have a characteristic taper at the anterior and posterior ends. The anterior is distinguished by an oral sucker at the point, an acetabulum and the testes. The posterior is where the uterus lies. In the parasite's midsection lie the vitelline glands that are involved in egg formation.
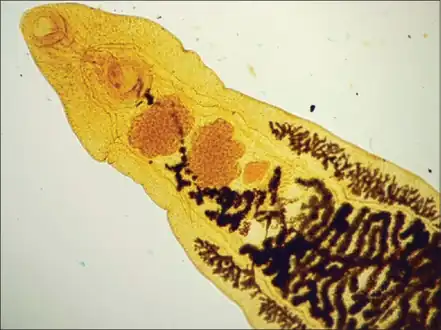 Structures at the anterior end of Dicrocoelium dendriticum
Structures at the anterior end of Dicrocoelium dendriticum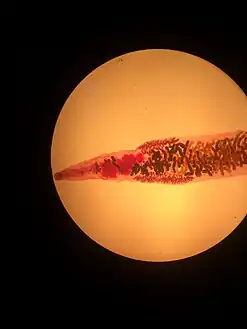 Mature Dicrocoelium dendriticum
Mature Dicrocoelium dendriticum
Life cycle
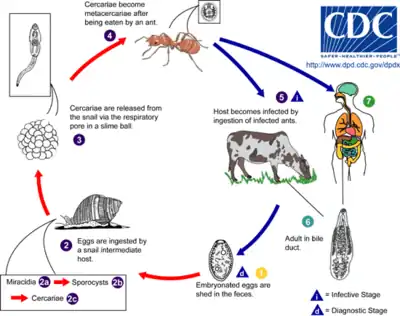
Dicrocoelium dendriticum spends its adult life inside the liver of its host. After mating, the eggs are excreted in the feces.
The first intermediate host, the terrestrial snail (Cochlicopa lubrica in the United States), consumes the feces, and becomes infected by the larval parasites. The larvae (or miracidium) drill through the wall of the gut and settle in its digestive tract, where they develop into a juvenile stage. The snail attempts to defend itself by walling the parasites off in cysts, which it then excretes and leaves behind in the grass or substrate.
The second intermediate host, an ant (Formica fusca in the United States[2]), uses the trail of snail slime as a source of moisture. The ant then swallows a cyst loaded with hundreds of juvenile lancet flukes. The parasites enter the gut and then drift through its body.
Most of the cercariae encyst in the haemocoel of the ant and mature into metacercariae, but one moves to the sub-esophageal ganglion (a cluster of nerve cells underneath the esophagus). There, the fluke takes control of the ant's actions by manipulating these nerves.[3] As evening approaches and the air cools, the infected ant is drawn away from other members of the colony and upward to the top of a blade of grass. Once there, it clamps its mandibles onto the top of the blade and stays there until dawn. Afterward, it goes back to its normal activity at the ant colony. If the host ant were to be subjected to the heat of the direct sun, it would die along with the parasite.
Night after night, the ant goes back to the top of a blade of grass until a grazing animal comes along and eats the blade, ingesting the ant along with it, thus putting lancet flukes back inside their host. They live out their adult lives inside the animal, reproducing so that the cycle begins again.[4][5][6] Infected ants may contain 100 metacercariae, and a high percentage of ants may be infected. Typical infections in cattle may be in the tens of thousands of adult worms.[7]
Transmission
Due to the highly specific nature of this parasite's life cycle, human infections are generally rare. Ruminants such as cows and sheep are usually the definitive host, but other herbivorous mammals and humans can also serve as definitive hosts through ingestion of infected ants. One definitive case involved a man who ingested bottled water contaminated by infected ants.[8]
Reservoirs
The main reservoirs for Dicrocoelium dendriticum are sheep, cows, land snails and ants. However, Dicrocoelium dendriticum has also been found in goats, pigs and even llamas and alpacas.
Incubation period
The incubation period for Dicrocoelium dendriticum is currently unknown.
History
Much of what is presently known about Dicrocoelium dendriticum is the result of the work of the naturalist Wendell Krull.[9] While D. dendriticum was discovered by Rudolphi in 1819 and D. hospes was discovered by Loos in 1899, the full life cycle was not known until Krull and C.R. Mapes published a series of papers from 1951-1953 detailing their observations and experiments. It was known that D. dendriticum affected sheep, but everything else was a mystery. The first link in the chain was the discovery of the first intermediate host, the land snail Cochlicopa lubrica (synonym: Cionella lubrica).[10] Next came the discovery that the slime balls coughed up by the snails could be a potential method of transfer of the parasite.[11] Shortly thereafter, the ant Formica fusca was found to be the second intermediate host by which sheep were infected.[12] Their work is the foundation of modern understanding of the parasite.
In humans
Dicrocoelium dendriticum along with Dicrocoelium hospes are part of a group of flukes that can infect the bile ducts of humans. Because the bodies of these parasites are long and narrow, infections are generally confined to the more distal parts of the bile ducts. As a result, most Dicrocoelium dendriticum infections of the biliary tree produce only mild symptoms. These symptoms can include biliary colic and general digestive disturbances, including bloating and diarrhea. However, in heavier infections, bile ducts and the biliary epithelium may become enlarged in addition to the generation of fibrous tissue surrounding the ducts, and as a result, causing an enlarged liver (hepatomegaly) or inflammation of the liver (cirrhosis).[13] In one unique case, an infection with Dicrocoelium dendriticum was associated with a skin rash urticaria.[14]
Diagnosis
Traditionally, diagnosis for dicrocoeliasis infection involves the identification of Dicrocoelium dendriticum eggs in the faeces of a human or other animal. However, in humans, eggs in the stool may be a result of ingesting raw infected animal liver and may not in fact indicate dicrocoeliasis.[15] Therefore, examining bile or duodenal fluid for eggs is a more accurate diagnostic technique in combination with a liver-free diet.[13]
In animals, diagnosis has traditionally involved stool examination or post-mortem examination of the liver. Recently, an ELISA using a Dicrocoelium dendriticum antigen was able to identify cases of dicrocoeliasis in sheep in Italy 28 days earlier than traditional methods.[16]
Management
Because human infections with Dicrocoelium dendriticum are so rare, there are multiple suggestions for treatment. The standard treatment is an anthelmintic such as Praziquantel, Triclabendazole, or Mirazid.
Epidemiology
Dicrocoeliasis is believed to be endemic or potentially endemic in 30 countries. Dicrocoelium dendriticum is found throughout Europe (former U.S.S.R., Switzerland, Italy, Germany, Spain, Turkey), the Middle East (Iran), Asia (China, Japan, Vietnam), Africa (Ghana, Nigeria, Sierra Leone) and in North and South America and Australia. The parasite tends to be found in areas that favor the intermediate hosts, such as fields with dry, chalky and alkaline soils.
In ruminants
Ruminants are the main definitive host of this fluke but other herbivorous animals, carnivores, and humans can be accidental definitive host.[15] Most infections, especially in cows, are asymptomatic but the effect on the liver depends on the number of flukes and the length of infection.[15][17] Since the fluke migrates up the biliary duct — but does not penetrate the gut wall or liver tissue — long infections may cause hypertrophy of the bile duct and liver lesion, even in the absence of symptoms.[17][16] While infections with D. dendriticum are usually symptom free, some animals may show anemia, edema, emaciation, and liver cirrhosis.[16] However, many of the symptoms of dicroceliosis are similar to those of other gastro-, intestinal-, and lung-nematode infections.
The diagnosis of D. dendriticum flukes is mainly from the recovery of adults in liver during necropsy or detecting eggs in animal feces.[16]
There is some evidence connecting decreased liver function from the trematode infection with pregnancy toxaemia and mastitis in ewes when combined with other risk factors.[18]
Treatment can be difficult due to the fluke's complex life-cycle. Various antihelminths, especially Netobimin, have been shown to be effective treatment when an entire herd is infected.[16] Animal husbandry practices can decrease the incidence of infection. This includes the avoidance of animal grazing early in the day or late in the evening, when ants are more likely to climb to the top of the grass blade.[16]
Public health prevention strategies
Current public health prevention strategies have involved the condemnation of contaminated livers so as to eliminate any possibility for food-borne infection.
In addition, in 2007 the World Health Organization included Dicrocoelium dendriticum on its list of organisms to target with its Foodborne Disease Burden Epidemiology Reference Group.
In addition, a study completed in Sweden combining data about the Dicrocoelium dendriticum prevalence and landscape data to discover in which landscape the parasite thrives. It was found that grazing land near forest areas (good for mollusks) and dry pastures with little other biodiversity (good for ants) both increased parasite prevalence.[19]
References
- ↑ "The Merckt Veterinary Manual". Merck & Co., Inc. 2008. Archived from the original on 2008-02-21. Retrieved 2008-07-03.
- ↑ "Dicrocoelium dendriticum is a bile duct fluke of ruminants such as sheep". California University of Pennsylvania. Archived from the original on 2008-08-20. Retrieved 2009-01-09.
- ↑ "A Fluke of Nature". Damn Interesting. Archived from the original on 2009-07-21. Retrieved 2007-03-22.
- ↑ "Dicrocoelium Dendriticum, the Liver Fluke". The Exile. December 12, 2003. Archived from the original on 2011-06-15. Retrieved 2008-07-03.
- ↑ Callahan, Gerald N. (November 24, 2002). "Infectious Madness: Disease with a Past and a Purpose: Mental illness may not be just craziness, but have a parasitic, fungal, or viral etiology". Emergency Medicine News. Archived from the original on July 29, 2012. Retrieved 2008-07-03.
- ↑ Holmes, Bob (November 6, 1993). "Evolution's neglected superstars: There is nothing glamorous about fleas, flukes or intestinal worms. So why are they suddenly attracting so much attention?". New Scientist. Archived from the original on 2008-07-09. Retrieved 2008-07-03.
- ↑ "University of Alberta Parasites Lab". University of Alberta. Archived from the original on 2008-05-17. Retrieved 2008-11-07.
- ↑ Drabick JJ, Egan JE, Brown SL, Vick RG, Sandman BM, Neafie RC (1988). "Dicroceliasis (lancet fluke disease) in an HIV seropositive man". JAMA. 259 (4): 567–8. doi:10.1001/jama.1988.03720040059028. PMID 3336179.
- ↑ Esch, Gerald (2007). Parasites and Infectious Disease Discovery by Serendipity and Otherwise (Parasites and Infectious Disease). New York: Cambridge UP. ISBN 978-0-521-85882-3.
- ↑ Mapes CR, Krull WH (October 1951). "Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica (Müller). II. Collection of the snail, Cionella lubrica, and its maintenance in the laboratory". The Cornell Veterinarian. 41 (4): 433–44. PMID 14887340.
- ↑ Krull WH, Mapes CR (April 1952). "Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica (Müller). III. Observations on the slimeballs of Dicrocoelium dendriticum". The Cornell Veterinarian. 42 (2): 253–76. PMID 14926337.
- ↑ Krull WH, Mapes CR (October 1952). "Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica (Müller). VII. The second intermediate host of Dicrocoelium dendriticum". The Cornell Veterinarian. 42 (4): 603–4. PMID 12998377.
- 1 2 Cengiz ZT, Yilmaz H, Dulger AC, Cicek M (2010). "Human infection with Dicrocoelium dendriticum in Turkey". Annals of Saudi Medicine. 30 (2): 159–61. doi:10.4103/0256-4947.60525. PMC 2855070. PMID 20220269.
- ↑ Sing A, Tybus K, Fackler I (2008). "Acute urticaria associated with Dicrocoelium dendriticum infestation". Indian Journal of Medical Microbiology. 26 (1): 97–8. doi:10.4103/0255-0857.38879. PMID 18227619. Archived from the original on 2021-09-16. Retrieved 2023-08-18.
- 1 2 3 "Dicrocoeliasis [Dicrocoelium dendriticum]". DPDx - Laboratory Identification of Parasites of Public Health Concern. Centers for Disease Control and Prevention. 28 December 2017. Archived from the original on 14 January 2019. Retrieved 13 January 2019.
- 1 2 3 4 5 6 Otranto, D; Traversa, D (August 2002). "A review of dicrocoeliosis of ruminants including recent advances in the diagnosis and treatment". Veterinary Parasitology. 107 (4): 317–35. doi:10.1016/s0304-4017(02)00121-8. PMID 12163243.
- 1 2 "2. The epidemiology of helminth parasites". www.fao.org. Archived from the original on 2007-07-13. Retrieved 2023-08-18.
- ↑ Mavrogianni, VS; Papadopoulos, E; Spanos, SA; et al. (February 2014). "Trematode infections in pregnant ewes can predispose to mastitis during the subsequent lactation period". Research in Veterinary Science. 96 (1): 171–9. doi:10.1016/j.rvsc.2013.11.009. PMID 24331730.
- ↑ Ekstam B, Johansson B, Dinnétz P, Ellström P (November 2011). "Predicting risk habitats for the transmission of the small liver fluke, Dicrocoelium dendriticum to grazing ruminants". Geospatial Health. 6 (1): 125–31. doi:10.4081/gh.2011.164. PMID 22109870.
{{cite journal}}: CS1 maint: url-status (link)
External links
- Lancet Fluke (Dicrocoelium lanceolatum) at The Living World of Mollusks
- Cartoon illustration of the cycle of Dicrocoelium dendriticum. Archived 2023-04-25 at the Wayback Machine