Onchocerca volvulus
| Onchocerca volvulus | |
|---|---|
 | |
| Onchocerca volvulus, the causative agent of river blindness | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Chromadorea |
| Order: | Rhabditida |
| Family: | Onchocercidae |
| Genus: | Onchocerca |
| Species: | O. volvulus |
| Binomial name | |
| Onchocerca volvulus Leuckart, 1893[1] | |
Onchocerca volvulus is a filarial (arthropod-borne) nematode (roundworm) that causes onchocerciasis (river blindness), and is the second-leading cause of blindness due to infection worldwide after trachoma. It is one of the 20 neglected tropical diseases listed by the World Health Organization, with elimination from certain countries expected by 2025.[2]
John O’Neill, an Irish surgeon, first described Onchocerca volvulus in 1874, when he found it to be the causative agent of ‘craw-craw’, a skin disease found in West Africa.[3] A Guatemalan doctor, Rodolfo Robles, first linked it to visual impairment in 1917.[4]
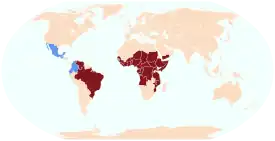
O. volvulus is primarily found in sub-Saharan Africa, and humans are the only known definitive host; there is also disease transmission in some South American nations, as well as Yemen (see global map bottom right). It is spread from person to person via female biting blackflies of the genus Simulium.[5]
Morphology
O. volvulus parasites obtain nutrients from the human host by ingesting blood or by diffusion through their cuticle. They may be able to trigger blood-vessel formation because dense vascular networks are often found surrounding the worms.[6] They are distinguished from other human-infecting filarial nematodes by the presence of deep transverse striations.[7]
It is a dioecious species, containing distinct males and females, which form nodules under the skin in humans. Mature female worms permanently reside in these fibrous nodules, while male worms are free to move around the subcutaneous tissue. The males are smaller than females, with male worms measuring 23 mm in length compared to 230–700 mm in females.[7]
The release of oocytes (eggs) in female worms does not depend upon the presence of a male worm, although they may attract male worms using unidentified pheromones.[8] The first larval stage, microfilariae, are 300 μm in length and unsheathed, meaning that when they mature into microfilariae, they exit from the envelope of the egg.[9]
Lifecycle
The average adult worm lifespan is 15 years, and mature females can produce between 500 and 1,500 microfilariae per day. The normal microfilarial lifespan is 1.0 to 1.5 years; however, their presence in the bloodstream causes little to no immune response until death or degradation of the microfilariae or adult worms.[10]
Blackfly stages
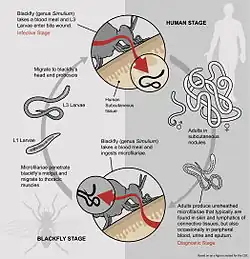
- The microfilariae of O. volvulus are found in the dermis layer of skin in the host.
- When a female Simulium blackfly takes a blood meal from an infected host, the microfilariae are also ingested.
- From here, the microfilariae penetrate the gut and migrate to the thoracic flight muscles, where they enter the first juvenile phase, J1.
- After maturing into J2, the second juvenile phase, they migrate to the proboscis, where they are found in the saliva.
- J2 stage juveniles then mature into infectious stage three juveniles, J3, in the saliva. The lifecycle in the blackfly takes between one and three weeks.[11]
Human stages
- When the female blackfly takes a blood meal, J3 juveniles pass into the human bloodstream.
- From here, the juveniles migrate to the subcutaneous tissue, where they form nodules and mature into adult worms over a period of 6–12 months.
- After maturation, the smaller adult males migrate from nodules to subcutaneous tissue, where they mate with the larger adult females.
- The eggs mature internally to form stage-one microfilariae, which are released from the female's body one at a time and remain in the subcutaneous tissue.
- The microfilariae are taken up by a female blackfly when it takes a blood meal, thus completing the lifecycle of O. volvulus.[5]
Genome

The total genome size of O. volvulus is 1.5x108 base pairs and contains around 4,000 genes, with genes for collagen and cuticular proteins being highly expressed in mature adults.[12]
O. volvulus has four chromosome pairs, which include a single pair of sex chromosomes. A large X sex chromosome and a smaller Y sex chromosome determine male worms, while two X chromosomes determine female worms.[13]
One of the three nonsex chromosomes is thought to have formed by a fusion event between two smaller chromosomes.[12]
Evolution
(Simplified phylogenetic tree of the genus Onchocerca.[14])
| |||||||||||||||||||||||||||||||||||||||||||
O. volvulus has low genetic variation between individuals. This suggests a population bottleneck occurred in the past that caused a rapid decrease in the population size.[12] It also shows high haplotype diversity, which is a measure of how unique a group of linked genes is. This pattern of low genetic variation and high haplotype diversity suggests fast population expansion after a bottleneck and has led to the theory that a host shift event from cattle allowed O. volvulus to infect humans.[15] This is also supported by genetic data that place O. ochengi (a cattle-infecting strain) as the sister group to O. volvulus.[14]
Endosymbiotic relationship with Wolbachia
O. volvulus, along with most filarial nematodes, share an endosymbiotic relationship with the bacterium Wolbachia. In the absence of Wolbachia, larval development of O. volvulus is disrupted or ceased.[16] These bacteria have been proposed to enhance the symptoms and severity of onchocerciasis by triggering inflammatory responses in the host.[17]
Disease
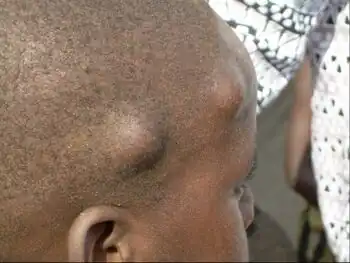
O. volvulus causes onchocerciasis, which causes severe itching. Long-term infection can cause keratitis, an inflammation of the cornea in the eye, and ultimately leads to blindness.[9] Symptoms are caused by the microfilariae and the immune response to infection, rather than the adults themselves. The most effective treatment involves using ivermectin, although resistance to this drug has been reported as developing.[18] Ivermectin prevents female worms from releasing microfilariae for several months, thus relieving symptoms and temporarily preventing transmission. However, this does not kill adult worms, so it must be taken once annually as long as adult worms are present.[19]
O. volvulus has been proposed as one of the causative agents of nodding syndrome, a condition that affects children aged 5 to 15 and is currently only observed in South Sudan, Tanzania, and northern Uganda. Although the cause of the disease is unknown, O. volvulus is being increasingly studied as a possible cause due to its ubiquity in areas where the disease is found.[20]
Immune response
Adult worms are found in nodules and are hidden from most components of the human immune system. Microfilariae are more vulnerable to attack by immune cells because they exit nodules to complete their lifecycle. O. volvulus can be detected by the immune system through the release of soluble antigens and antigens found on the surface of microfilariae and infective J3 juveniles. These antigens allow the immune system to detect the presence of a foreign organism in the body and trigger an immune response to clear infection.[21]
The immune response involves raising antibodies (IgG, IgM and IgE type) that can react with soluble antigens released by Onchocerca volvulus.[22] Opsonising antibodies that tag cells for destruction are also found against the infective J3 stage and microfilariae, but there is not enough evidence at the moment to say whether this is protective.[23]
The antigens of O. volvulus are highly complex and show cross-reactivity with several other filarial worms. Little evidence indicates that antibodies made are specific to O. volvulus. However, after the age of 40, the number of parasites carried (the intensity of infection) decreases, suggesting that over time, some sort of protective immune response develops.[21]
Modulation by O. volvulus
Microfilariae can also modulate the immune system to avoid destruction. The complement system is used to enhance the effect of antibodies and phagocytic cells, which engulf and destroy other cells. Microfilariae block this pathway by cleaving C3b—an important protein in this process—to form iC3b. iC3b cannot go on to activate the next step in the pathway and allows microfilariae to remain in the body with little to no attack by the immune system.[24]
Epidemiology
An estimated 187 million people are at risk of O. volvulus infection, with 17–25 million people infected and 0.8 million showing some impairment of vision. O. volvulus has not directly caused a single death, but has cost 1.1 million disability adjusted life years, which measure the number of years of healthy life lost due to a specific disease and show the burden of a disease.[26]
Simulium blackfly adults require moving water to breed and eggs remain in water until they exit from the pupa and enter the adult stage of their lifecycle. Due to this restriction, O. volvulus is only found around streams or rivers. Artificial water systems, such as hydroelectric power plants, built in Africa, provide ideal conditions all year for blackfly development and make controlling its spread difficult.[27]
About 99% of cases of onchocerciasis are found in 31 countries in sub-Saharan Africa, although areas of limited transmission occur in Brazil, Venezuela, and Yemen.[25] The disease is thought to have been imported into Latin America through the slave trade.[28] Onchocerciasis was eliminated from Colombia in 2013, Ecuador in 2014, Mexico in 2015, and Guatemala in 2016[26] due to control programs that used mass drug administration with ivermectin.[25]
References
- ↑ "Onchocerca volvulus Leuckart, 1893". Global Biodiversity Information Facility. Retrieved 7 June 2023.
- ↑ "Neglected tropical diseases". World Health Organization. March 2017. Archived from the original on 22 February 2014. Retrieved 17 March 2017.
- ↑ O’Neill, J. (1875). "On the presence of a filaria in " craw-craw" (PDF). The Lancet. 105 (2686): 265–266. doi:10.1016/s0140-6736(02)30941-3. Archived from the original (PDF) on 2 February 2016. Retrieved 7 June 2023.
- ↑ Robles, R. (1917). "Enfermedad nueva en Guatemala". La Juventud Médica.
- 1 2 Duke, B.O. (1993). "The population dynamics of Onchocerca volvulus in the human host". Tropical Medicine and Parasitology. 44 (2): 61–68. ISSN 0177-2392. PMID 8367667.
- ↑ Burnham, G. (1998). "Onchocerciasis". Lancet. 351 (9112): 1341–1346. doi:10.1016/S0140-6736(97)12450-3. ISSN 0140-6736. PMID 9643811. S2CID 208794023.
- 1 2 Neafie, R.C. (1972). "Morphology of Onchocerca volvulus". American Journal of Clinical Pathology. 57 (5): 574–586. doi:10.1093/ajcp/57.5.574. ISSN 0002-9173. PMID 5025601.
- ↑ Schulz-Key, H.; Soboslay, P.T. (1994). "Reproductive biology and population dynamics of Onchocerca volvulus in the vertebrate host". Parasite. 1 (1S): S53–S55. doi:10.1051/parasite/199401s1053. ISSN 1252-607X.
- 1 2 Udall, D.N. (2007). "Recent updates on onchocerciasis: diagnosis and treatment". Clinical Infectious Diseases. 44 (1): 53–60. doi:10.1086/509325. ISSN 1537-6591. PMID 17143815.
- ↑ Schulz-Key, H. (1990). "Observations on the Reproductive Biology of Onchocerca volvulus". Acta Leidensia (in Nederlands). 59 (1–2): 27–44. PMID 2378210. Archived from the original on 4 September 2023. Retrieved 7 June 2023.
- ↑ Eichner, M.; Renz, A.; Wahl, G.; Enyong, P. (1991). "Development of Onchocerca volvulus microfilariae injected into Simulium species from Cameroon". Medical and Veterinary Entomology. 5 (3): 293–298. doi:10.1111/j.1365-2915.1991.tb00555.x. ISSN 1365-2915. PMID 1768922. S2CID 2794018.
- 1 2 3 Unnasch, Thomas R; Williams, S.A. (2000). "The genomes of Onchocerca volvulus". International Journal for Parasitology. 30 (4): 543–552. doi:10.1016/S0020-7519(99)00184-8. PMID 10731575.
- ↑ Post, R. (2005). "The chromosomes of the Filariae". Filaria Journal. 4: 10. doi:10.1186/1475-2883-4-10. ISSN 1475-2883. PMC 1282586. PMID 16266430.
- 1 2 team, European Centre for Disease Prevention and Control (ECDC)—Health Communication Unit—Eurosurveillance editorial (23 April 2015). "Human case of Onchocerca lupi infection, Germany, August 2014". Eurosurveillance. 20 (16). doi:10.2807/1560-7917.es2015.20.16.21099. PMID 25953271.
- ↑ Morales-Hojas, R.; Cheke, R.A.; Post, R.J. (2007). "A preliminary analysis of the population genetics and molecular phylogenetics of Onchocerca volvulus (Nematoda: Filarioidea) using nuclear ribosomal second internal transcribed spacer sequences". Memórias do Instituto Oswaldo Cruz. 102 (7): 879–882. doi:10.1590/S0074-02762007005000114. ISSN 0074-0276. PMID 17992364.
- ↑ Saint André, A.V.; Blackwell, N.M.; Hall, L.R.; Hoerauf, A.; Brattig, N.W.; Volkmann, L.; Taylor, M.J.; Ford, L.; Hise, A.G. (2002). "The Role of Endosymbiotic Wolbachia Bacteria in the Pathogenesis of River Blindness" (PDF). Science. 295 (5561): 1892–1895. Bibcode:2002Sci...295.1892S. doi:10.1126/science.1068732. ISSN 0036-8075. PMID 11884755. S2CID 22130066. Archived (PDF) from the original on 4 September 2023. Retrieved 7 June 2023.
- ↑ Tamarozzi, F.; Halliday, A.; Gentil, K.; Hoerauf, A.; Pearlman, E.; Taylor, M.J. (2011). "Onchocerciasis: the Role of Wolbachia Bacterial Endosymbionts in Parasite Biology, Disease Pathogenesis, and Treatment". Clinical Microbiology Reviews. 24 (3): 459–468. doi:10.1128/CMR.00057-10. ISSN 0893-8512. PMC 3131055. PMID 21734243.
- ↑ Lustigman, S.; McCarter, J.P. (2007). "Ivermectin Resistance in Onchocerca volvulus: Toward a Genetic Basis". PLOS Neglected Tropical Diseases. 1 (1): e76. doi:10.1371/journal.pntd.0000076. ISSN 1935-2735. PMC 2041823. PMID 17989789.
- ↑ Ejere, Henry O. D.; Schwartz, Ellen; Wormald, Richard; Evans, Jennifer R. (15 August 2012). "Ivermectin for onchocercal eye disease (river blindness)". The Cochrane Database of Systematic Reviews (8): CD002219. doi:10.1002/14651858.CD002219.pub2. ISSN 1469-493X. PMC 4425412. PMID 22895928.
- ↑ Idro, R.; Opar, B.; Wamala, J.; Abbo, C.; Onzivua, S.; Mwaka, D.A.; Kakooza-Mwesige, A.; Mbonye, A.; Aceng, J.R. (2016). "Is nodding syndrome an Onchocerca volvulus-induced neuroinflammatory disorder? Uganda's story of research in understanding the disease". International Journal of Infectious Diseases. 45: 112–117. doi:10.1016/j.ijid.2016.03.002. ISSN 1878-3511. PMID 26987477.
- 1 2 Greene, B.M.; Gbakima, A.A.; Albiez, E.J.; Taylor, H.R. (1985). "Humoral and cellular immune responses to Onchocerca volvulus infection in humans". Reviews of Infectious Diseases. 7 (6): 789–795. doi:10.1093/clinids/7.6.789. ISSN 0162-0886. PMID 4070916.
- ↑ Ngu, J.L.; Blackett, K. (1976). "Immunological studies in onchocerciasis in Cameroon". Tropical and Geographical Medicine. 28 (2): 111–120. ISSN 0041-3232. PMID 788262.
- ↑ Greene, B.M.; Taylor, H.R.; Aikawa, M. (1981). "Cellular killing of microfilariae of Onchocerca volvulus: eosinophil and neutrophil-mediated immune serum-dependent destruction". Journal of Immunology. 127 (4): 1611–1618. ISSN 0022-1767. PMID 7276574.
- ↑ Meri, T.; Jokiranta, T.S.; Hellwage, J.; Bialonski, A.; Zipfel, P.F.; Meri, S. (2002). "Onchocerca volvulus microfilariae avoid complement attack by direct binding of factor H". The Journal of Infectious Diseases. 185 (12): 1786–1793. doi:10.1086/340649. ISSN 0022-1899. PMID 12085326.
- 1 2 3 "Onchocerciasis Fact sheet N°374". World Health Organization. March 2017. Retrieved 16 March 2017.
- 1 2 "Progress towards eliminiating onchocerciasis in the WHO region of the Americas: Verification of elimination of transmission in Guatemala and progress report on the elimination of human onchocerciasis, 2015-2016". World Health Organization. March 2017. Archived from the original on 23 March 2017. Retrieved 17 March 2017.
- ↑ Myburgh, E.; Nevill, E.M. (2003). "Review of blackfly (Diptera: Simuliidae) control in South Africa". The Onderstepoort Journal of Veterinary Research. 70 (4): 307–316. doi:10.4102/ojvr.v70i4.295. ISSN 0030-2465. PMID 14971733.
- ↑ Gustavsen, K.; Hopkins, A.; Sauerbrey, M. (2011). "Onchocerciasis in the Americas: from arrival to (near) elimination". Parasites & Vectors. 4: 205. doi:10.1186/1756-3305-4-205. ISSN 1756-3305. PMC 3214172. PMID 22024050.
External links
- "Onchocerca volvulus". NCBI Taxonomy Browser. 6282. Archived from the original on 7 September 2023. Retrieved 7 June 2023.