Strongyloides stercoralis
| Threadworm | |
|---|---|
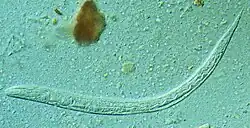 | |
| First stage larva (L1) of S. stercoralis | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Chromadorea |
| Order: | Rhabditida |
| Family: | Strongylidae |
| Genus: | Strongyloides |
| Species: | S. stercoralis |
| Binomial name | |
| Strongyloides stercoralis Bavay, 1876 | |
Strongyloides stercoralis is a human pathogenic parasitic roundworm causing the disease strongyloidiasis. Its common name in the US is threadworm. In the UK and Australia, however, the term threadworm can also refer to nematodes of the genus Enterobius, otherwise known as pinworms.[1]
The Strongyloides stercoralis nematode can parasitize humans. The adult parasitic stage lives in tunnels in the mucosa of the small intestine. The genus Strongyloides contains 53 species,[2][3] and S. stercoralis is the type species. S. stercoralis has been reported in other mammals, including cats and dogs. However, it seems that the species in dogs is typically not S. stercoralis, but the related species S. canis. Non-human primates are more commonly infected with S. fuelleborni and S. cebus, although S. stercoralis has been reported in captive primates. Other species of Strongyloides that are naturally parasitic in humans, but with restricted distributions, are S. fuelleborni in central Africa and S. kellyi in Papua New Guinea.
Life cycle
The life cycle of this parasite is more complex than that of most nematodes, with its alternation between free-living and parasitic cycles, and its potential for autoinfection (the parasite has the ability to complete its life cycle without the involvement of another host) and multiplication within the host. The parasitic cycle is homogonic, while the free-living cycle is heterogonic. The heterogonic life cycle is advantageous to the parasite because it allows reproduction in the absence of a host.
In the free-living cycle, the rhabditiform larvae passed in the stool can either molt twice and become infective filariform larvae (direct development) or molt four times and become free-living adult males and females that mate and produce eggs from which rhabditiform larvae hatch. In the direct development, first-stage larvae (L1) transform into infective larvae (IL) via three molts. The indirect route results first in the development of free-living adults that mate; the female lays eggs, which hatch and then develop into IL. The direct route gives IL faster (three days) versus the indirect route (seven to 10 days). However, the indirect route results in an increase in the number of IL produced. Speed of development of IL is traded for increased numbers. The free-living males and females of S. stercoralis die after one generation; they do not persist in the soil. The latter, in turn, can either develop into a new generation of free-living adults or develop into infective filariform larvae. The filariform larvae penetrate the human host skin to initiate the parasitic cycle. Upon contact with contaminated soil, infectious larvae contained in the soil can penetrate the skin. While S. stercoralis is attracted to chemicals such as carbon dioxide or sodium chloride, these chemicals are not specific. Larvae have been thought to locate their hosts via chemicals in the skin, the predominant one being urocanic acid, a histidine metabolite on the uppermost layer of skin that is removed by sweat or the daily skin-shedding cycle.[4] Urocanic acid concentrations can be up to five times greater in the foot than any other part of the human body. Some of them enter the superficial veins and are carried in the blood to the lungs, where they enter the alveoli. They are then coughed up and swallowed into the gut, where they parasitise the intestinal mucosa of the duodenum and jejunum. In the small intestine, they molt twice and become adult female worms. The females live threaded in the epithelium of the small intestine and, by parthenogenesis, produce eggs, which yield rhabditiform larvae. Only females will reach reproductive adulthood in the intestine. Female strongyloids reproduce through parthenogenesis. The eggs hatch in the intestine and young larvae are then excreted in the feces. It takes about two weeks to reach egg development from the initial skin penetration. By this process, S. stercoralis can cause both respiratory and gastrointestinal symptoms. The worms also participate in autoinfection, in which the rhabditiform larvae become infective filariform larvae, which can penetrate either the intestinal mucosa (internal autoinfection) or the skin of the perianal area (external autoinfection); in either case, the filariform larvae may follow the previously described route, being carried successively to the lungs, the bronchial tree, the pharynx, and the small intestine, where they mature into adults; or they may disseminate widely in the body. To date, occurrence of autoinfection in humans with helminthic infections is recognized only in Strongyloides stercoralis and Capillaria philippinensis infections. In the case of Strongyloides, autoinfection may explain the possibility of persistent infections for many years in persons not having been in an endemic area and of hyperinfections in immunodepressed individuals.
Zoonotic transmission
Dogs can act as a host for this parasite both in the wild and in the laboratory but transmission from dog to human has been difficult to prove. Molecular genetic analyses have shown that there are two populations of this parasite in dogs, one of which (type B) is exclusive to dogs and a second (type A) that is common to dogs and humans.[5][6] These two genotypes may be separate species. The identity of the genes suggests that dog to human transmission may occur.
Morphology
Whereas males grow to only about 0.9 mm (0.04 in) in length, females can grow from 2.0 to 2.5 mm (0.08 to 0.10 in). Both sexes also possess a tiny buccal capsule and cylindrical esophagus without a posterior bulb.[7] In the free-living stage, the esophagi of both sexes are rhabditiform. Males can be distinguished from females by two structures: the spicules and gubernaculum.
Autoinfection
An unusual feature of S. stercoralis is autoinfection. Only one other species in the genus Strongyloides, S. felis, has this trait. Autoinfection is the development of L1 into small infective larvae in the gut of the host. These autoinfective larvae penetrate the wall of the lower ileum or colon or the skin of the perianal region, enter the circulation again, travel to the lungs, and then to the small intestine, thus repeating the cycle. Autoinfection makes strongyloidiasis due to S. stercoralis an infection with several unusual features.
Persistence of infection is the first of these important features. Because of autoinfection, humans have been known to still be infected up to 65 years after they were first exposed to the parasite (e.g., World War II or Vietnam War veterans). Once a host is infected with S. stercoralis, infection is lifelong unless effective treatment eliminates all adult parasites and migrating autoinfective larvae.
Geographic distribution
S. stercoralis infection is associated with fecal contamination of soil or water. Hence, it is a very rare infection in developed economies. In developing countries, it is less prevalent in urban areas than in rural areas (where sanitation standards are poor). S. stercoralis can be found in areas with tropical and subtropical climates.[8]
Strongyloidiasis was first described in the 19th century in French soldiers returning home from expeditions in Indochina. Today, the countries of the old Indochina (Vietnam, Cambodia, and Laos) still have endemic strongyloidiasis, with the typical prevalences being 10% or less. Regions of Japan used to have endemic strongyloidiasis, but control programs have eliminated the disease. Strongyloidiasis appears to have a high prevalence in some areas of Brazil and Central America. It is endemic in Africa, but the prevalence is typically low (1% or less). Pockets have been reported from rural Italy, but the current status is unknown. In the Pacific islands, strongyloidiasis is rare, although some cases have been reported from Fiji. In tropical Australia, some rural and remote Australian Aboriginal communities have very high prevalences of strongyloidiasis.[9]
In some African countries (e.g., Congo), S. fuelleborni was more common than S. stercoralis in parasite surveys from the 1970s, but the current status is unknown. In Papua New Guinea, S. stercoralis is endemic, but prevalence is low. However, in some areas, another species, S. kellyi,[10] is a very common parasite of children in the New Guinea Highlands and Western Province.[10]
Knowledge of the geographic distribution of strongyloidiasis is of significance to travelers who may acquire the parasite during their stays in endemic areas.
Because strongyloidiasis could theoretically be transmittable through unsanitary bedclothes care must be taken never to use unclean hotel bed sheets in endemic areas. Using plastic slippers when showering may be very important when travelling in tropical regions.
Estimates of the number of people infected vary with one estimate putting the figure at 370 million worldwide.[11][12] Local prevalence can exceed 40% in some tropical and subtropical countries.[13]
Disease
Symptoms and signs
Many people infected are asymptomatic at first. Symptoms include dermatitis: swelling, itching, larva currens, and mild hemorrhage at the site where the skin has been penetrated. Spontaneous scratch-like lesions may be seen on the face or elsewhere. If the parasite reaches the lungs, the chest may feel as if it is burning, and wheezing and coughing may result, along with pneumonia-like symptoms (Löffler's syndrome). The intestines could eventually be invaded, leading to burning pain, tissue damage, sepsis, and ulcers. Stools may have yellow mucus with a recognizable smell. Chronic diarrhea can be a symptom.[14] In severe cases, edema may result in obstruction of the intestinal tract, as well as loss of peristaltic contractions.[15]
Strongyloidiasis in immunocompetent individuals is usually an indolent disease. However, in immunocompromised individuals, it can cause a hyperinfective syndrome (also called disseminated strongyloidiasis) due to the reproductive capacity of the parasite inside the host. This hyperinfective syndrome can have a mortality rate close to 90% if disseminated.[16][17][18]
Immunosuppressive drugs, especially corticosteroids and agents used for tissue transplantation, can increase the rate of autoinfection to the point where an overwhelming number of larvae migrate through the lungs, which in many cases can prove fatal. In addition, diseases such as human T-lymphotropic virus 1, which enhance the Th1 arm of the immune system and lessen the Th2 arm, increase the disease state.[17] Another consequence of autoinfection is the autoinfective larvae can carry gut bacteria back into the body. About 50% of people with hyperinfection present with bacterial disease due to enteric bacteria. Also, a unique effect of autoinfective larvae is larva currens due to the rapid migration of the larvae through the skin. Larva currens appears as a red line that moves rapidly (more than 5 cm or 2 in per day), and then quickly disappears. It is pathognomonic for autoinfective larvae and can be used as a diagnostic criterion for strongyloidiasis due to S. stercoralis.
Diagnosis
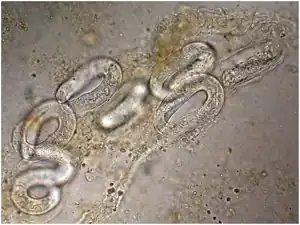
Locating juvenile larvae, either rhabditiform or filariform, in recent stool samples will confirm the presence of this parasite.[19] Other techniques used include direct fecal smears, culturing fecal samples on agar plates, serodiagnosis through ELISA, and duodenal fumigation. Still, diagnosis can be difficult because of the day-to-day variation in juvenile parasite load.
Prevention
Ideally, prevention, by improved sanitation (proper disposal of feces), practicing good hygiene (washing of hands), etc., is used before any drug regimen is administered.
Treatment
Ivermectin is the drug of choice for treatment, due to its low side effect profile.[20] Albendazole is also effective in treating strongyloidiasis. Mebendazole has a much higher failure rate in clinical practice than albendazole or ivermectin.[21] However, these drugs have little effect on the autoinfective larvae. Hence, repeat treatments with ivermectin or albendazole must be administered to kill newly matured parasites that have developed from the autoinfective larvae. This means a full treatment dose every two weeks until all larvae capable of maturing into adults have been extirpated. Follow-up stool samples, potential additional treatment, and blood tests are necessary to ensure a cure.[22]
Chemoattractant
This parasite depends on chemical cues to find a potential host. It uses sensor neurons of class AFD to identify cues excreted by the host.[23] S. stercoralis is attracted to nonspecific attractants of warmth, carbon dioxide, and sodium chloride. Urocanic acid, a component of skin secretions in mammals, is a major chemoattractant. Larvae of S. stercoralis are strongly attracted to this compound.[4] This compound can be suppressed by metal ions, suggesting a possible strategy for preventing infection.
See also
References
- ↑ Vanderkooi, M. (2000). Village Medical Manual (5th ed.). Pasadena: William Carey Library. ISBN 0878087788.
- ↑ Speare, R. (1989). "Identification of species of Strongyloides". In Grove, D. I. (ed.). Strongyloidiasis: a major roundworm infection of man. London: Taylor & Francis. pp. 11–83. ISBN 0850667321.
- ↑ Skerratt, L. F. (1995). "Strongyloides spearei n. sp. (Nematoda: Strongyloididae) from the common wombat Vombatus ursinus (Marsupialia: Vombatidae)". Systematic Parasitology. 32 (2): 81–89. doi:10.1007/BF00009506. S2CID 45308882.
- 1 2 Safer, D.; Brenes, M.; Dunipace, S.; Schad, G. (2007). "Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis". Proceedings of the National Academy of Sciences. 104 (5): 1627–30. Bibcode:2007PNAS..104.1627S. doi:10.1073/pnas.0610193104. PMC 1785286. PMID 17234810.
- ↑ Nagayasu, Eiji; Thet Hnin Htwe Aung, Myo Pa Pa; Hortiwakul, Thanaporn; Hino, Akina; Tanaka, Teruhisa; Higashiarakawa, Miwa; Olia, Alex; Tomoyo, Taniguchi; Thu Win, Soe Moe; Ohashi, Isao; Odongo-Aginya, Emmanuel I.; Aye, Khin Myo; Mon, Mon; Win, Kyu Kyu; Ota, Kei; Torisu, Yukari; Panthuwong, Siripen; Kimura, Eisaku; Palacpac, Nirianne M.Q.; Kikuchi, Taisei; Hirata, Tetsuo; Torisu, Shidow; Hisaeda, Hajime; Horii, Toshihiro; Fujita, Jiro; Htike, Wah Win; Maruyama, Haruhiko (2017). "A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis, unveiled by molecular phylogeny". Scientific Reports. 7 (1): 4844. Bibcode:2017NatSR...7.4844N. doi:10.1038/s41598-017-05049-x. PMC 5501853. PMID 28687738.
- ↑ Jaleta, Tegegn G.; Zhou, Siyu; Bemm, Felix M.; Schär, Fabian; Khieu, Virak; Muth, Sinuon; Odermatt, Peter; Lok, James B.; Streit, Adrian (2017). "Different but overlapping populations of Strongyloides stercoralis in dogs and humans—Dogs as a possible source for zoonotic strongyloidiasis". PLOS Neglected Tropical Diseases. 11 (8): e0005752. doi:10.1371/journal.pntd.0005752. PMC 5565190. PMID 28793306.
- ↑ Roberts, L.; Janovy, J. Jr. (2005). Foundations of Parasitology (7th ed.). Boston: McGraw Hill. p. 412. ISBN 0071112715.
- ↑ Segarra-Newnham, M. (2007). "Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection". Ann Pharmacother. 41 (12): 1992–2001. doi:10.1345/aph.1K302. PMID 17940124. S2CID 38184274.
- ↑ Johnston, F. H.; Morris, P. S.; Speare, R.; McCarthy, J.; Currie, B.; Ewald, D.; Page, W.; Dempsey, K. (2005). "Strongyloidiasis: A review of the evidence for Australian practitioners". The Australian Journal of Rural Health. 13 (4): 247–54. doi:10.1111/j.1440-1584.2005.00710.x. PMID 16048468.
- 1 2 Dorris, M.; Viney, M. E.; Blaxter, M. L. (2002). "Molecular phylogenetic analysis of the genus Strongyloides and related nematodes". International Journal for Parasitology. 32 (12): 1507–17. doi:10.1016/s0020-7519(02)00156-x. PMID 12392916.
- ↑ Olsen, A; Van Lieshout, L; Marti, H; Polderman, T; Polman, K; Steinmann, P; Stothard, R; Thybo, S; Verweij, J. J.; Magnussen, P (2009). "Strongyloidiasis—the most neglected of the neglected tropical diseases?" (PDF). Transactions of the Royal Society of Tropical Medicine and Hygiene. 103 (10): 967–72. doi:10.1016/j.trstmh.2009.02.013. PMID 19328508. Archived (PDF) from the original on 2022-12-25. Retrieved 2022-11-22.
- ↑ Schär, F; Trostdorf, U; Giardina, F; Khieu, V; Muth, S; Marti, H; Vounatsou, P; Odermatt, P (2013). "Strongyloides stercoralis: Global Distribution and Risk Factors". PLOS Neglected Tropical Diseases. 7 (7): e2288. doi:10.1371/journal.pntd.0002288. PMC 3708837. PMID 23875033.
- ↑ Laymanivong, S; Hangvanthong, B; Insisiengmay, B; Vanisaveth, V; Laxachack, P; Jongthawin, J; Sanpool, O; Thanchomnang, T; Sadaow, L; Phosuk, I; Rodpai, R; Maleewong, W; Intapan, P. M. (2016). "First molecular identification and report of genetic diversity of Strongyloides stercoralis, a current major soil-transmitted helminth in humans from Lao People's Democratic Republic". Parasitology Research. 115 (8): 2973–80. doi:10.1007/s00436-016-5052-z. PMID 27083185. S2CID 18567198.
- ↑ Thamwiwat, Alisa; Mejia, Rojelio; Nutman, Thomas B.; Bates, Jeffrey T. (6 July 2014). "Strongyloidiasis as a Cause of Chronic Diarrhea, Identified Using Next-Generation Strongyloides stercoralis-Specific Immunoassays". Current Tropical Medicine Reports. 1 (3): 145–147. doi:10.1007/s40475-014-0026-7.
- ↑ Roberts, L.; Janovy, J. Jr. (2005). Foundations of Parasitology (7th ed.). Boston: McGraw Hill. pp. 414–415. ISBN 0071112715.
- ↑ Igra-Siegman, Y; Kapila, R; Sen, P; Kaminski, ZC; Louria, DB (1981). "Syndrome of hyperinfection with Strongyloides stercoralis". Reviews of Infectious Diseases. 3 (3): 397–407. doi:10.1093/clinids/3.3.397. PMID 7025145.
- 1 2 Marcos, L. A.; Terashima, A.; Dupont, H. L.; Gotuzzo, E. (2008). "Strongyloides hyperinfection syndrome: An emerging global infectious disease". Transactions of the Royal Society of Tropical Medicine and Hygiene. 102 (4): 314–318. doi:10.1016/j.trstmh.2008.01.020. PMID 18321548.
- ↑ Newberry, AM; Williams, DN; Stauffer, WM; Boulware, DR; Hendel-Paterson, BR; Walker, PF (Nov 2005). "Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis". Chest. 128 (5): 3681–4. doi:10.1378/chest.128.5.3681. PMC 1941746. PMID 16304332.
- ↑ Roberts, L.; Janovy, J. Jr. (2005). Foundations of Parasitology (7th ed.). Boston: McGraw Hill. p. 415. ISBN 0071112715.
- ↑ Luvira, Viravarn; Watthanakulpanich, Dorn; Pittisuttithum, Punnee (2014-08-30). "Management of Strongyloides stercoralis: a puzzling parasite". International Health. Oxford University Press (OUP). 6 (4): 273–281. doi:10.1093/inthealth/ihu058. ISSN 1876-3413. PMID 25173343.
- ↑ Boulware, DR; Stauffer, WM; Hendel-Paterson, BR; Rocha, JL; Seet, RC; Summer, AP; Nield, LS; Supparatpinyo, K; Chaiwarith, R; Walker, PF (June 2007). "Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey". The American Journal of Medicine. 120 (6): 545.e1–8. doi:10.1016/j.amjmed.2006.05.072. PMC 1950578. PMID 17524758.
- ↑ PEARSON.RICHARD (2018-08-31). "Strongyloidiasis – Infectious Diseases". Merck Manuals Professional Edition. Archived from the original on 2018-09-03. Retrieved 2018-09-03.
- ↑ Forbes, WM; Ashton, FT; Boston, R; Zhu, X; Schad, GA (2004). "Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively". Veterinary Parasitology. 120 (3): 189–98. doi:10.1016/j.vetpar.2004.01.005. PMID 15041094.
External links
- Overview of taxonomy of genus Strongyloides.
- The Australian Society for Parasitology Archived 2022-11-15 at the Wayback Machine