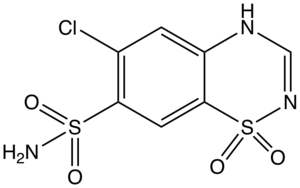
Chlorothiazide
 | |
 | |
| Names | |
|---|---|
| Pronunciation | klor" oh thye' a zide[1] |
| Trade names | Diuril, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Thiazide diuretic[1] |
| Main uses | High blood pressure, swelling[2] |
| Side effects | Nausea, dizziness, headache, increased urination, dehydration, dry mouth, low sodium, low potassium, low magnesium[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, IV |
| Typical dose | 500 to 1000 mg OD or BID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682341 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | low |
| Metabolism | Nil |
| Elimination half-life | 45 to 120 minutes |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C7H6ClN3O4S2 |
| Molar mass | 295.71 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Chlorothiazide, sold under the brand name Diuril among others, is a medication used to treat high blood pressure and swelling.[2] It is one of a number of first line options for high blood pressure.[2] It may be used for swelling due to heart failure, nephrotic syndrome, or pregnancy.[2] It may be taken by mouth or by injection into a vein.[2]
Common side effects include nausea, dizziness, headache, increased urination, dehydration, dry mouth, low sodium, low potassium, and low magnesium.[1] Other side effects may include allergic reactions, gout, and low blood pressure.[2] While safety is unclear; it has been used as a second line treatment to control blood pressure and swelling in pregnancy.[2][3] It is a thiazide diuretic.[1]
Chlorothiazide was patented in 1956 and approved for medical use in 1958.[4] It is on the World Health Organization's List of Essential Medicines as an alternative to hydrochlorothiazide.[5] It is available as a [[generic medication].[6] In the United States 90 tablets of 500 mg costs about 14 USD.[6]
Medical uses
- Large amount of excess fluid including:
Dosage
The recommended dose by mouth in adults is 500 to 1000 mg once or twice daily.[1] It is also available as an injection in vials of 500 mg.[1]
Contraindications
Side effects
History
Marketing of chlorothiazide began in 1957.[8] The research team of Merck Sharp and Dohme Research Laboratories of Beyer, Sprague, Baer, and Novello created a new series of medications, the thiazide diuretics, which includes chlorothiazide. They won an Albert Lasker Special Award in 1975 for this work.[9]
The structure has been determined by X-ray crystallography.[10]
See also
References
- 1 2 3 4 5 6 7 8 "Thiazide Diuretics". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 15 May 2021. Retrieved 4 January 2022.
- 1 2 3 4 5 6 7 "Chlorothiazide Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 4 January 2022.
- ↑ "Chlorothiazide Use During Pregnancy". Drugs.com. Archived from the original on 24 October 2020. Retrieved 4 January 2022.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 456. ISBN 9783527607495. Archived from the original on 2021-11-01. Retrieved 2021-10-04.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- 1 2 "Chlorothiazide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 30 April 2016. Retrieved 4 January 2022.
- 1 2 "Diuril (Chlorothiazide): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Archived from the original on 2019-10-07. Retrieved 2019-10-07.
- ↑ Eknoyan, Garabed (1997). "1. A history of diuretics". In Seldin, Donald W.; Giebisch, Gerhard H. (eds.). Diuretic Agents: Clinical Physiology and Pharmacology. San Diego: Academic Press. p. 25. ISBN 0-12-635690-4. Archived from the original on 2021-11-13. Retrieved 2021-11-13.
- ↑ "Historical Awards - The Lasker Foundation". The Lasker Foundation. Archived from the original on 2015-12-23. Retrieved 2021-10-04.
- ↑ Johnston A, Bardin J, Johnston BF, Fernandes P, Kennedy AR, Price SL, Florence AJ (2011). "Experimental and Predicted Crystal Energy Landscapes of Chlorothiazide". Crystal Growth & Design. 11 (2): 405–413. doi:10.1021/cg1010049.
External links
| Identifiers: |
|---|