Etanercept
 | |
| Names | |
|---|---|
| Trade names | Enbrel, Benepali, Erelzi, others |
| Other names | Etanercept-szzs, etanercept-ykro |
| Clinical data | |
| Main uses | Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | Subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602013 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 58–76% (SC) |
| Metabolism | Reticuloendothelial system (speculative) |
| Elimination half-life | 70–132 hours |
| Chemical and physical data | |
| Formula | C2224H3475N621O698S36 |
| Molar mass | 51235.07 g·mol−1 |
Etanercept, sold under the brand name Enbrel among others, is a medication used to treat autoimmune diseases including rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, plaque psoriasis, and ankylosing spondylitis.[1] It is given by injection under the skin.[3]
Common side effects include pain at the site of injection and infections.[1] Other side effects may include cancers, heart failure, demyelinating diseases, allergic reactions, and pancytopenia.[4] Safety in pregnancy is unclear.[4] It is a TNF inhibitor which blocks tumor necrosis factor (TNF).[1] It a monoclonal antibody made by recombinant DNA techniques.[1][4]
Etanercept was approved for medical use in the United States in 1998 and Europe in 2000.[4][1] It is on the World Health Organization's List of Essential Medicines as an alternative to adalimumab.[5] In the United Kingdom 4 weeks of medication costs the NHS about £715 as of 2021.[3] This amount in the United States costs about 5,700 USD.[6]
Medical uses
In the United States etanercept is indicated for:
- Moderate to severe rheumatoid arthritis (RA) (Nov 1998)[7]
- Moderate to severe polyarticular juvenile rheumatoid arthritis (May 1999)[8]
- Psoriatic arthritis (Jan 2002)[9]
- Ankylosing spondylitis (AS) (July 2003)[10][11]
- Moderate to severe plaque psoriasis (April 2004)[12]
In the European Union etanercept is indicated to treat:
- moderate to severe active rheumatoid arthritis[13]
- severe, active and progressive rheumatoid arthritis[13]
- juvenile idiopathic arthritis[13]
- polyarthritis (rheumatoid-factor-positive or -negative) and extended oligoarthritis in children and adolescents[13]
- active and progressive psoriatic arthritis[13]
- enthesitis-related arthritis
- axial spondyloarthritis[13]
- severe active ankylosing spondylitis[13]
- severe non-radiographic axial spondyloarthritis[13]
- moderate to severe plaque psoriasis[13]
- chronic severe plaque psoriasis pediatric plaque psoriasis[13]
Dosage
It is generally given at a dose of 25 mg twice per week or 50 mg once per week.[3] Though occasionally 50 mg twice per week may be used.[3]
Side effects
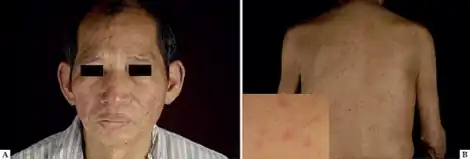
In 2008, the FDA placed a black box warning on etanercept due to a number of serious infections associated with the drug.[14] Serious infections and sepsis, including fatalities, have been reported with use including reactivation of latent tuberculosis and hepatitis B infections.[15][16]
There has also been a report of strongyloides hyperinfection after use of etanercept.[17]
Mechanism of action
It reduces the effect of naturally present TNF, and hence is a TNF inhibitor, functioning as a decoy receptor that binds to TNF.[18]
Tumor necrosis factor-alpha (TNFα) is a cytokine produced by lymphocytes and macrophages, two types of white blood cells. It mediates the immune response by attracting additional white blood cells to sites of inflammation and through additional molecular mechanisms that initiate and amplify inflammation. Inhibition of its action by etanercept reduces the inflammatory response, which is especially useful for treating autoimmune diseases.
There are two types of TNF receptors: those found embedded in white blood cells that respond to TNF by releasing other cytokines, and soluble TNF receptors that are used to deactivate TNF and blunt the immune response. In addition, TNF receptors are found on the surface of virtually all nucleated cells (red blood cells, which are not nucleated, do not contain TNF receptors on their surface). Etanercept mimics the inhibitory effects of naturally occurring soluble TNF receptors, the difference being that etanercept, because it is a fusion protein rather than a simple TNF receptor, has a greatly extended half-life in the bloodstream, and therefore a more profound and long-lasting biologic effect than a naturally occurring soluble TNF receptor.[19]
Chemistry
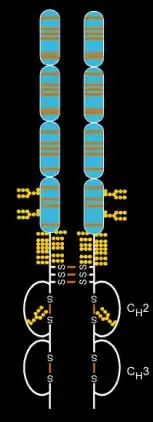
Etanercept is made from the combination of two naturally occurring soluble human 75-kilodalton TNF receptors linked to an Fc portion of an IgG1.[20] The effect is an artificially engineered dimeric fusion protein.[20] Etanercept is a complex molecule containing 6 N-glycans, up to 14 O-glycans and 29 disulfide bridge structures.[21][22][23]
It fuses the TNF receptor to the constant end of the IgG1 antibody. First, the developers isolated the DNA sequence that codes the human gene for soluble TNF receptor 2, which is a receptor that binds to tumor necrosis factor-alpha. Second, they isolated the DNA sequence that codes the human gene for the Fc end of immunoglobulin G1 (IgG1). Third, they linked the DNA for TNF receptor 2 to the DNA for IgG1 Fc. Finally, they expressed the linked DNA to produce a protein that links the protein for TNF receptor 2 to the protein for IgG1 Fc.
History
The prototypic fusion protein was first synthesized and shown to be highly active and unusually stable as a modality for blockade of TNF in vivo in the early 1990s by Bruce A. Beutler, and his colleagues.[24][25] The first etanercept-related patent was filed by Immunex on September 5, 1989.[26]
Etanercept was approved for use in the United States in November 1998.[27]
Etanercept was approved for use in the European Union in February 2000.[13]
Society and culture
Cost
The cost of the medication in the U.S. is $6,240 (USD) for 4 ml of subcutaneous solution 50 mg/mL[28]
.svg.png.webp) Etanercept costs (US)
Etanercept costs (US).svg.png.webp) Etanercept prescriptions (US)
Etanercept prescriptions (US)
The U.S. retail price of Enbrel has risen over time. In 2008, the cost of Enbrel was $1,500 per month or $18,000 per year.[29] By 2011, the cost had exceeded $20,000 per year.[30][31] In 2013, a survey by the International Federation of Health Plans (IFHP) found that the average U.S. cost for Enbrel was $2,225 per month, or $26,700 per year.[32] The IFHP report also found wide variation in prices charged to various U.S. health plans, between $1,946 per month at the 25th percentile and $4,006 per month at the 95th percentile.[32]
Enbrel is more expensive in the U.S. than in other countries.[32] As of 2013, average monthly costs in surveyed nations ranged from $1,017 in Switzerland to $1,646 in Canada, compared to an average monthly cost of $2,225 per month in the U.S.[32]
Amgen sells Enbrel within the U.S. and Canada, while Pfizer, Inc. sells the drug outside of the U.S. and Canada.[30] Sales within the U.S. and Canada were $3.5 billion in 2010.[30] Sales of Enbrel outside the U.S. and Canada were $3.3 billion in 2010.[33]
These investigators also patented the protein,[34] selling all rights to its use to Immunex, a biotechnology company that was acquired by Amgen in 2002.[35]
Marketing
In North America, etanercept is marketed by Amgen under the trade name Enbrel in two separate formulations, one in powder form, the other as a pre-mixed liquid. Wyeth (now part of Pfizer) was the sole marketer of Enbrel outside North America excluding Japan where Takeda Pharmaceuticals markets the drug.
Patents
The patent on Enbrel was originally set to expire on October 23, 2012,[36] but, in the United States, a second patent, granting exclusivity for another 16 years, has been granted.[37]
Before the extension it seemed unlikely that a generic would have been available. As a biologic, etanercept is subject to different laws from those applicable to chemical formulations. Currently many countries do not permit the manufacture of generic biologics. However, the European Union and the United States (Biologics Price Competition and Innovation Act of 2009 Archived 2019-03-02 at the Wayback Machine) do currently have in place a system to approve generic biologics (biosimilars) which "requires mandatory clinical testing and periodic review".[38]
In April 2013, the Indian pharma major Cipla made an announcement about launching the first biosimilar of Etanercept in India under the brand name 'Etacept' for the treatment of rheumatic disorders. The company's April 17, 2013 press release claimed that the biosimilar will cost 30% less as compared to the innovator.[39]
In January 2015, Samsung and Biogen's joint venture "Samsung Bioepis" successfully submitted "Benepali", a biosimilar version of the drug, for review to the European Medicines Agency (EMA), and announced that it will seek regulatory approvals in other territories as well. Later the same year, the European Medicines Agency also accepted Sandoz’s application for review of its etanercept biosimilar "Erelzi" which will be marketed by Novartis.[40]
In the U.S., Sandoz submitted a biologics license application (BLA) for the proposed etanercept product "GP2015" in July 2016. Upon acceptance of the first application process, the U.S. FDA reviewed data from European clinical trials and bio-analytical investigations, demonstrating the biosimilarity of GP2015 to the US-licensed Enbrel. Sandoz in 2009 tried to invalidate the patents held by Hoffman-La Roche/Immunex and exclusively licensed to Amgen but lost in federal court. Sandoz was then countersued by Amgen for patent infringements related to the methods of treating psoriasis and/or psoriatic arthritis. The case Immunex Corp. et al. vs. Sandoz Inc. et al., 16-cv-01118-CCC-JBC (D.N.J.) is pending.[41]
Biosimilars
In January 2016, Benpali was approved for use in the European Union.[42]
In February 2017, Lifmior was approved for use in the European Union.[43] It was withdrawn from the market in February 2020.[44]
In June 2017, Erelzi was approved for use in the European Union.[45]
In May 2020, Nepexto was approved for use in the European Union.[46]
Similar agents
- Soluble TNF receptor
- Anti-TNF monoclonal antibodies
References
- 1 2 3 4 5 6 "Enbrel". Archived from the original on 12 November 2020. Retrieved 16 December 2021.
- 1 2 "Etanercept Use During Pregnancy". Drugs.com. 24 January 2020. Archived from the original on 20 January 2021. Retrieved 13 August 2020.
- 1 2 3 4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1161. ISBN 978-0857114105.
- 1 2 3 4 "Etanercept Monograph for Professionals". Drugs.com. Archived from the original on 28 September 2021. Retrieved 16 December 2021.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Etanercept Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 11 January 2022. Retrieved 16 December 2021.
- ↑ Siegel JP (November 2, 1998). "Approval of Etanercept for treatment of rheumatoid arthritis" (PDF). Letter to Sally Gould. Food and Drug Administration. Retrieved April 14, 2015.
- ↑ Weiss, Karen D. (May 27, 1999). "Approval of Etanercept for treatment of polyarticular course juvenile rheumatoid arthritis (JRA)" (PDF). Letter to Sally Gould. Food and Drug Administration. Retrieved April 14, 2015.
- ↑ Weiss KD (January 15, 2002). "Approval of Etanercept for treatment of psoriatic arthritis". Letter to Sally Gould. Food and Drug Administration. Retrieved April 14, 2015.
- ↑ Keegan P (July 24, 2003). "Approval of Etanercept for treatment of ankylosing spondylitis" (PDF). Letter to Douglas Hunt. Food and Drug Administration. Retrieved April 14, 2015.
- ↑ Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, Benkhalti Jandu M, Tugwell P, Wells GA (April 2015). "TNF-alpha inhibitors for ankylosing spondylitis". The Cochrane Database of Systematic Reviews. 4 (4): CD005468. doi:10.1002/14651858.CD005468.pub2. PMID 25887212. Archived from the original on 2020-09-20. Retrieved 2021-01-07.
- ↑ Walton M (April 30, 2004). "Approval of Etanercept for treatment of moderate to severe plaque psoriasis" (PDF). Letter to Douglas Hunt. Food and Drug Administration. Retrieved April 14, 2015.
- 1 2 3 4 5 6 7 8 9 10 11 "Enbrel EPAR". European Medicines Agency (EMA). Archived from the original on 12 November 2020. Retrieved 2 April 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Wyeth and Amgen heighten warning of life-threatening infections on skin drug Enbrel". Archived from the original on 2008-05-05. Retrieved 2008-05-02.
- ↑ Safety Update on TNF- α Antagonists: Infliximab and Etanercept (PDF). Food and Drug Administration. pp. 13–14. Archived (PDF) from the original on 24 September 2015. Retrieved 20 December 2013.
- ↑ "Prescribing Information – Enbrel". Archived from the original on 2007-10-14. Retrieved 2008-01-10.
- ↑ "UpToDate". Archived from the original on 2021-01-06. Retrieved 2021-01-07.
- ↑ Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O'Brien C, Eivazi A, Kung J, Nguyen DH, Doberstein SK, Erard F, Ryffel B, Szymkowski DE (August 2007). "Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection". Journal of Immunology. 179 (3): 1872–83. doi:10.4049/jimmunol.179.3.1872. PMID 17641054.
- ↑ Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, Hoare S, Balkwill F, Ganesan TS (September 2005). "Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer". Journal of Clinical Oncology. 23 (25): 5950–59. doi:10.1200/JCO.2005.04.127. PMID 16135466.
- 1 2 Smola MG, Soyer HP, Scharnagl E (October 1991). "Surgical treatment of dermatofibrosarcoma protuberans. A retrospective study of 20 cases with review of literature". European Journal of Surgical Oncology. 17 (5): 447–53. PMID 1936291.
- ↑ Houel S, Hilliard M, Yu YQ, McLoughlin N, Martin SM, Rudd PM, Williams JP, Chen W (January 2014). "N- and O-glycosylation analysis of etanercept using liquid chromatography and quadrupole time-of-flight mass spectrometry equipped with electron-transfer dissociation functionality". Analytical Chemistry. 86 (1): 576–84. doi:10.1021/ac402726h. PMID 24308717.
- ↑ Mukai Y, Nakamura T, Yoshikawa M, Yoshioka Y, Tsunoda S, Nakagawa S, Yamagata Y, Tsutsumi Y (November 2010). "Solution of the structure of the TNF-TNFR2 complex". Science Signaling. 3 (148): ra83. doi:10.1126/scisignal.2000954. PMID 21081755. S2CID 24226117.
- ↑ Lamanna WC, Mayer RE, Rupprechter A, Fuchs M, Higel F, Fritsch C, Vogelsang C, Seidl A, Toll H, Schiestl M, Holzmann J (June 2017). "The structure-function relationship of disulfide bonds in etanercept". Scientific Reports. 7 (1): 3951. Bibcode:2017NatSR...7.3951L. doi:10.1038/s41598-017-04320-5. PMC 5479810. PMID 28638112.
- ↑ Peppel K, Crawford D, Beutler B (December 1991). "A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity". The Journal of Experimental Medicine. 174 (6): 1483–89. doi:10.1084/jem.174.6.1483. PMC 2119031. PMID 1660525.
- ↑ Peppel K, Poltorak A, Melhado I, Jirik F, Beutler B (November 1993). "Expression of a TNF inhibitor in transgenic mice". Journal of Immunology. 151 (10): 5699–703. PMID 7693816.
- ↑ Norman, Peter (February 16, 2017). "Enbrel and etanercept biosimilars: a tale of two patent system". Pharmaceutical Patent Analyst. 6 (1): 5–7. doi:10.4155/ppa-2016-0043. PMID 28201948.
- ↑ "Etanercept Product Approval Information - Licensing Action 12/2/98". U.S. Food and Drug Administration (FDA). 1 April 2016. Archived from the original on 18 January 2017. Retrieved 4 June 2020.
- ↑ "Enbrel Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 April 2021. Retrieved 30 March 2021.
- ↑ "What's behind the whopping price tags on the newest generation of drugs: Archived 2013-07-28 at the Wayback Machine The story behind the production of Enbrel, Amgen's popular rheumatoid arthritis drug, provides insights as to why bioengineered drugs are so expensive." Carol M. Ostrom, Seattle Times, August 18, 2008
- 1 2 3 "Patent for Amgen Drug May Undercut Health Care Plan" Archived 2021-07-25 at the Wayback Machine, Andrew Pollock, New York Times, November 23, 2011
- ↑ "Co-pay hike a painful reality; Miracle drug monthly cost jumps from $42 to $600" Archived 2013-01-18 at archive.today, Margery Eagan, Boston Herald, November 3, 2011
- 1 2 3 4 "2013 Comparative Price Report" (PDF). International Federation of Health Plans. Archived from the original (PDF) on 22 October 2017. Retrieved 24 November 2017.
- ↑ "Portions of the 2010 Financial Report". Sec.gov. Archived from the original on 2019-06-10. Retrieved 2019-06-05.
- ↑ U.S. Patent number: 5,447,851
- ↑ "Arthritis Drug Effective for Depression in Psoriasis Sufferers". Archived from the original on 2007-10-20. Retrieved 2008-01-10.
- ↑ "Patent Terms Extended Under 35 USC §156". Archived from the original on 2010-02-24. Retrieved 2021-01-07.
- ↑ "New Amgen Enbrel patent could block biosimilars until 2028". 2011-11-25. Archived from the original on 2019-07-14. Retrieved 2019-07-14.
- ↑ Kaldre I (2008). "The Future of Generic Biologics: Should the United States "Follow-On" the European Pathway?". www.law.duke.edu. Archived from the original on 2021-08-28. Retrieved 2019-06-05.
- ↑ "Cipla - Home" (PDF). Cipla.com. Archived from the original (PDF) on 2013-05-01. Retrieved 2019-06-05.
- ↑ Biosimilar etanercept submitted for approval in EU Archived 2020-11-26 at the Wayback Machine GaBI Online – Generics and Biosimilars Initiative (Jan 2015). Retrieved 13 July 2016.
- ↑ FDA Briefing Document Arthritis Advisory Committee Meeting July 13, 2016 Archived April 26, 2019, at the Wayback Machine. FDA AAC Brief. BLA 761042, GP2015, a proposed biosimilar to Enbrel. Retrieved 13 July 2016.
- ↑ "Benpali EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 30 December 2019. Retrieved 2 April 2020.
- ↑ "Lifmior EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 29 November 2020. Retrieved 2 April 2020.
- ↑ "Public statement on Lifmior: Withdrawal of the marketing authorisation in the European Union" (PDF). Archived (PDF) from the original on 28 August 2021. Retrieved 2 April 2020.
- ↑ "Erelzi EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 30 December 2019. Retrieved 2 April 2020.
- ↑ "Nepexto EPAR". European Medicines Agency (EMA). 24 March 2020. Archived from the original on 4 June 2020. Retrieved 4 June 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |