Guanidine
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Guanidine[1] | |||
| Other names
Iminomethanediamine | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
506044 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.656 | ||
| EC Number |
| ||
Gmelin Reference |
100679 | ||
| MeSH | Guanidine | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
CH5N3 | ||
| Molar mass | 59.072 g·mol−1 | ||
| Melting point | 50 °C (122 °F; 323 K) | ||
| log P | −1.251 | ||
| Conjugate acid | Guanidinium | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
−57 – −55 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.0511 – −1.0531 MJ mol−1 | ||
| Pharmacology | |||
| Pharmacokinetics: | |||
| 7–8 hours | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
475 mg/kg (oral, rat)[2] | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
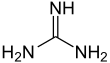
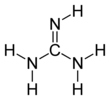
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine as a normal product of protein metabolism. A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine.
Structure
Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a NH
2 group.[3] Isobutylene can be seen as the carbon analogue in much the same way. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule.[4] In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction.[5]
Production
Guanidine can be obtained from natural sources, being first isolated by Adolph Strecker via the degradation of guanine.[6] It was first synthesized in 1861 by the oxidative degradation of an aromatic natural product, guanine, isolated from Peruvian guano.[7]
A laboratory method of producing guanidine is gentle (180-190 °C) thermal decomposition of dry ammonium thiocyanate in anhydrous conditions:
The commercial route involves a two step process starting with the reaction of dicyandiamide with ammonium salts. Via the intermediacy of biguanidine, this ammonolysis step affords salts of the guanidinium cation (see below). In the second step, the salt is treated with base, such as sodium methoxide.[6]
Chemistry
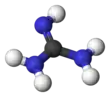
Guanidinium cation
With a pKb of 0.4, guanidine is a strong base. Most guanidine derivatives are in fact salts containing the conjugate acid.
The conjugate acid is called the guanidinium cation, (C(NH
2)+
3). This planar, symmetric ion consists of three amino groups each bonded to the central carbon atom with a covalent bond of order 4/3. It is a highly stable +1 cation in aqueous solution due to the efficient resonance stabilization of the charge and efficient solvation by water molecules. As a result, its pKaH is 13.6[8] meaning that guanidine is a very strong base in water; in neutral water, it exists almost exclusively as guanidinium.
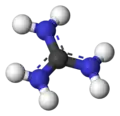
ball-and-stick model 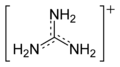
resonance hybrid 
canonical forms
Testing for guanidine
Guanidine can be selectively detected using sodium 1,2-naphthoquinone-4-sulfonic acid (Folin's reagent) and acidified urea.[9]
Uses
Industry
The main salt of commercial interest is the nitrate [C(NH
2)3]NO
3. It is used as a propellant, for example in air bags.
Medicine
Since the Middle Ages in Europe, guanidine has been used to treat diabetes as the active antihyperglycemic ingredient in French lilac. Due to its long-term hepatoxicity, further research for blood sugar control was suspended at first after the discovery of insulin. Later development of nontoxic, safe biguanides led to the long-used first-line diabetes control medicine metformin, introduced to Europe in the 1950s & United States in 1995 and now prescribed to over 17 million patients per year in the US.[10][11]
Guanidinium chloride[12] is a now-controversial adjuant in treatment of botulism. Recent studies have shown some significant subsets of patients who see no improvement after the administration of this drug.[13]
Biochemistry
Guanidine exists protonated, as guanidinium, in solution at physiological pH.
Guanidinium chloride (also known as guanidine hydrochloride) has chaotropic properties and is used to denature proteins. Guanidinium chloride is known to denature proteins with a linear relationship between concentration and free energy of unfolding. In aqueous solutions containing 6 M guanidinium chloride, almost all proteins lose their entire secondary structure and become randomly coiled peptide chains. Guanidinium thiocyanate is also used for its denaturing effect on various biological samples.
Other
Guanidinium hydroxide is the active ingredient in some non-lye hair relaxers.
Guanidine derivatives
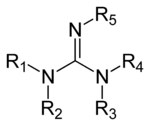
Guanidines are a group of organic compounds sharing a common functional group with the general structure (R
1R
2N)(R
3R
4N)C=N−R
5. The central bond within this group is that of an imine, and the group is related structurally to amidines and ureas. Examples of guanidines are arginine, triazabicyclodecene, saxitoxin, and creatine.
Galegine is an isoamylene guanidine.[14]
See also
- Category:Guanidines
- Sakaguchi test
- Y-aromaticity
- Amidine
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 883. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ "Guanidine hydrochloride". ChemIDplus. National Library of Medicine. Archived from the original on 2014-08-12. Retrieved 2014-08-10.
- ↑ Goebel, M.; Klapoetke, T. M. (2007). "First structural characterization of guanidine". Chem. Commun. 43 (30): 3180–2. doi:10.1039/B705100J. PMID 17653381.
- ↑ Yamada, T.; Liu, X.; Englert, U.; Yamane, H.; Dronskowski, R. (2009). "Solid-state structure of free base guanidine achieved at last". Chem. Eur. J. 15 (23): 5651–5. doi:10.1002/chem.200900508. PMID 19388036.
- ↑ Sawinski, P. K.; Meven, M.; Englert, U.; Dronskowski, R. (2013). "Single-Crystal Neutron Diffraction Study on Guanidine, CN3H5". Cryst. Growth Des. 13 (4): 1730–5. doi:10.1021/cg400054k.
- 1 2 Güthner, Thomas; Mertschenk, Bernd; Schulz, Bernd. "Guanidine and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_545.pub2.
- ↑ Strecker, A. (1861). "Untersuchungen über die chemischen Beziehungen zwischen Guanin, Xanthin, Theobromin, Caffeïn und Kreatinin" [Studies on the chemical relationships between guanine, xanthine, theobromine, caffeine and creatinine]. Liebigs Ann. Chem. 118 (2): 151–177. doi:10.1002/jlac.18611180203. Archived from the original on 2021-07-16. Retrieved 2019-07-02.
- ↑ Perrin, D. D. (1972). Dissociation Constants of Organic Bases in Aqueous Solution (Supplement ed.). London: Butterworths..
- ↑ Sullivan, M. X. (1935-10-01). "A Colorimetric Test for Guanidine". Proceedings of the Society for Experimental Biology and Medicine. 33 (1): 106–108. doi:10.3181/00379727-33-8270C. ISSN 0037-9727. S2CID 88290359.
- ↑ Blaslov, Kristina; Naranđa, Fran Stjepan; Kruljac, Ivan; Renar, Ivana Pavlić (2018-12-15). "Treatment approach to type 2 diabetes: Past, present and future". World Journal of Diabetes. 9 (12): 209–219. doi:10.4239/wjd.v9.i12.209. ISSN 1948-9358. PMC 6304295. PMID 30588282. Archived from the original on 2022-02-19. Retrieved 2022-02-17.
- ↑ "The Top 300 of 2019". clincalc.com. Archived from the original on 2021-02-12. Retrieved 2022-02-17.
- ↑ Blaslov, Kristina (2018). PMID 30588282.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ↑ Brook, Itzhak (2001). Pediatric Anaerobic Infections: Diagnosis and Management (3rd ed.). Taylor & Francis. p. 529. ISBN 0824741862.
- ↑ Witters, L. A. (2001). "The blooming of the French lilac". Journal of Clinical Investigation. 108 (8): 1105–7. doi:10.1172/JCI14178. PMC 209536. PMID 11602616.