Progressive vaccinia
| Progressive vaccinia | |
|---|---|
| Other names: Vaccinia gangrenosum, vaccinia necrosum, disseminated vaccinia | |
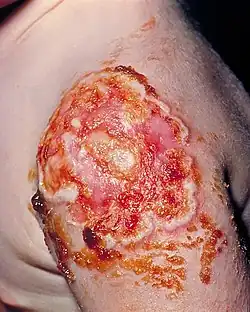 | |
| Necrotic vaccination site, due to the onset of progressive vaccinia | |
| Specialty | Dermatology |
| Symptoms | Malaise, fever, vomiting and tender, enlarged axillary lymph nodes; progresses to septic Pseudomonas aeruginosa, likely from a perirectal abscess, Clostridium difficile (bacteria), Staphylococcus aureus and cell-mediated immunodeficiency. |
| Complications | Necrosis of the injected part, exacerbating to gangrene and eventual amputation. Usually, the pocks tend to go away without scarring; however, the external and internal spread of the virus may have serious consequences in persons with eczema and other forms of atopic dermatitis, in these persons, defects of innate immunity and a high level of Th2 cell activity render the skin unusually permissive to the initiation and rapid spread of vaccinia infection (known as “eczema vaccinatum”)[1][2] |
| Usual onset | 11 days to 6.5 weeks |
| Duration | Long-lasting |
| Causes | Injection by the vaccinia virus[3] |
| Risk factors | People with cellular immunodeficiencies |
| Diagnostic method | Fever and headache, then progressive ulceration and necrosis of the injection site for smallpox, albeit the lack of inflammation is noted as the "hallmark of PV"[3] |
| Differential diagnosis | May initially be mistaken for leukemia |
| Prevention | Unknown |
| Treatment | Vaccinia Immune Globulin Intravenous (Human) (VIGIV), Emergency Investigational New Drug (E-IND) both administered orally and topically, (in this case ST-246); CMX001, a lipid conjugate of cidofovir and granulocyte colony-stimulating factor for the exiguous normal white blood cells;supportive care; skin graft |
| Medication | Imiquimod, and thiosemicarbazone |
| Prognosis | Lifelong |
| Frequency | 1 to 2 per million during routine vaccination during 1963-1968 for smallpox |
| Deaths | 15% risk of death |
Progressive vaccinia is a rare skin condition caused by the vaccinia virus, characterized by painless, but progressive, necrosis and ulceration.[4]
Signs and symptoms
Complications
Opportunistic fungal, protozoal, or bacterial infections and the vaccinia virus itself may lead to septic shock and disseminated intravascular coagulation, in addition to necrosis and ulcerated skin tissue. Some of these tissues may eventually become large, requiring not only a skin graft but surgical removal of the destroyed tissue, in order to avoid graft-versus-host disease in organ transplanted patients, in whom immunosuppressive therapy would otherwise have to be discontinued to allow healing of the wound.
Pathophysiology
Vaccinia is introduced into the skin by means of multiple punctures of a bifurcated needle. The virus replicates in the basal layer and disseminates from cell to cell, causing necrosis and the formation of fluid-filled vesicles. Nonetheless, the initial spread of the virus is slowed by innate antiviral mechanisms, and, by the second week, the cell-mediated immune response begins to eliminate infected cells[lower-alpha 1]. Neutrophils, macrophages, and lymphocytes infiltrate the inoculation site, forming a confluent pustule and releasing cytokines and chemokines that cause hyperemia and edema in surrounding tissues. This may initially manifest into complaints of malaise and other mild constitutional symptoms, fever, vomiting, and tender enlarged axillary lymph nodes. Some vaccinees develop additional local “satellite” pustules that resolve along with the primary lesion.
The virus may gain access to the blood at an early stage, and secondary skin lesions, which follow the same evolution as the inoculation site, may appear across the body. Bacteria, like Staphylococcus aureus, may infect the ulcerated, and necrotic lesions. Coalescent lesions may cover large portions of the body with extensive tissue destruction. Although some vaccinia viruses commonly disseminate through the bloodstream, the NYCBOH strain reportedly causes only limited viremia in a small percentage of recipients during the period of pustule formation.[6][7] The inflammatory process reaches its peak by days 10–12 after vaccination and begins to resolve by day 14, with the shedding of the scab and other pustules by day 21. This sequence of events, which simulates the development of smallpox "pock", is known as a “take” reaction. A successful "take" is required for the development of antivaccinia antibody and cell-mediated responses.[8][9][10][11]
Treatment
In addition to a skin graft, some medications also work. Among antiviral substances, cidofovir showed some effect in preliminary studies. Apart from treating opportunistic infections with anti-viral, and antibiotic medications, for HIV or immunocompromised (or at the very least iatrogenic immunosuppression like cancer and autoimmune disease) people, immediate highly active antiretroviral therapy (HAART) in HIV patients and withdrawal of immunosuppressive therapy accompanied by aggressive administration of VIG are given to save the patient's life. Intensive care and supportive treatment are required. VIG is given at up to 10 ml / kg body weight.
Note
- ↑ Immunosuppressed individuals tend to have a larger fatality rate and tendency to get the virus due to HIV infection, iatrogenic immunosuppression, etc. Although these conditions are contraindications to the dermovaccine, inadvertent inoculation after contact with a vaccinee may occur; in layman's terms, inoculation means the introduction of a pathogen or antigen into a living organism to stimulate the production of antibodies.[5] Due to the impaired immune response of the host, the virus multiplies by cell-to-cell spread at the inoculation site, and the lesion expands circumferentially, forming the trademark symptoms called "pocks".
References
- ↑ Copeman, P. W. M.; Wallace, H. J. (10 October 1964). "Eczema Vaccinatum". BMJ. 2 (5414): 906–908. doi:10.1136/bmj.2.5414.906. PMC 1816899. PMID 14185655.
- ↑ Engler, Renata J.M.; Kenner, Julie; Leung, Donald Y.M. (September 2002). Written at Walter Reed Army Medical Center, Allergy-Immunology Department, Washington, DC, USA. "Smallpox vaccination: Risk considerations for patients with atopic dermatitis". Journal of Allergy and Clinical Immunology. Maryland Heights, Missouri, United States: jacionline.org. 110 (3): 357–365. doi:10.1067/mai.2002.128052. PMID 12209080.
- 1 2 Centers for Disease Control Prevention (CDC) (22 May 2009). "Progressive Vaccinia in a Military Smallpox Vaccinee – United States, 2009". Morbidity and Mortality Weekly Report. 58 (19): 532–6. PMID 19478722. Archived from the original on 1 March 2021. Retrieved 25 May 2021.
- ↑ James, William D.; Elston, Dirk; Treat, James R.; Rosenbach, Misha A.; Neuhaus, Isaac (2020). "19. Viral diseases". Andrews' Diseases of the Skin: Clinical Dermatology (13th ed.). Edinburgh: Elsevier. pp. 387–388. ISBN 978-0-323-54753-6. Archived from the original on 2022-06-03. Retrieved 2022-05-30.
- ↑ "inoculation". Merriam-Webster Dictionary. Retrieved December 9, 2020.
- ↑ Fenner, Frank; Henderson, Donald A.; Arita, Isao; Jezek, Zdenek; Ladnyi, Ivan Danilovich; Organization, World Health (1988). Smallpox and its eradication. World Health Organization. hdl:10665/39485. ISBN 978-92-4-156110-5.
- ↑ Blattner, Russell J.; Norman, James O.; Heys, Florence M.; Aksu, Ismet (June 1964). "Antibody response to cutaneous inoculation with vaccinia virus: Viremia and viruria in vaccinated children". The Journal of Pediatrics. 64 (6): 839–852. doi:10.1016/s0022-3476(64)80642-9. PMID 14172233.
- ↑ Frey, Sharon E.; Couch, Robert B.; Tacket, Carol O.; Treanor, John J.; Wolff, Mark; Newman, Frances K.; Atmar, Robert L.; Edelman, Robert; Nolan, Carrie M.; Belshe, Robert B.; National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group (25 April 2002). "Clinical Responses to Undiluted and Diluted Smallpox Vaccine". New England Journal of Medicine. 346 (17): 1265–1274. doi:10.1056/NEJMoa020534. PMID 11923490.
- ↑ Frey, Sharon E.; Newman, Frances K.; Cruz, John; Shelton, W. Brian; Tennant, Janice M.; Polach, Tamara; Rothman, Alan L.; Kennedy, Jeffrey S.; Wolff, Mark; Belshe, Robert B.; Ennis, Francis A. (25 April 2002). "Dose-Related Effects of Smallpox Vaccine". New England Journal of Medicine. 346 (17): 1275–1280. doi:10.1056/NEJMoa013431. PMID 11923489. Archived from the original on 26 September 2020. Retrieved 25 May 2021.
- ↑ Ennis, Francis A.; Cruz, John; Demkowicz, Jr., Walter E.; Rothman, Alan L.; McClain, David J. (June 2002). "Primary Induction of Human CD8 + Cytotoxic T Lymphocytes and Interferon‐γ–Producing T Cells after Smallpox Vaccination". The Journal of Infectious Diseases. 185 (11): 1657–1659. doi:10.1086/340517. PMID 12023773.
- ↑ McClain, David J.; Harrison, Shannon; Yeager, Curtis L.; Cruz, John; Ennis, Francis A.; Gibbs, Paul; Wright, Michael S.; Summers, Peter L.; Arthur, James D.; Graham, Jess A. (April 1997). "Immunologic Responses to Vaccinia Vaccines Administered by Different Parenteral Routes". The Journal of Infectious Diseases. 175 (4): 756–763. doi:10.1086/513968. PMID 9086127.