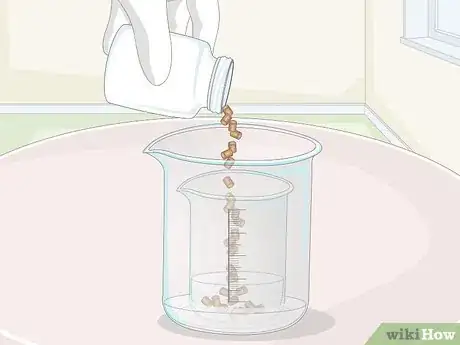This article was co-authored by wikiHow Staff. Our trained team of editors and researchers validate articles for accuracy and comprehensiveness. wikiHow's Content Management Team carefully monitors the work from our editorial staff to ensure that each article is backed by trusted research and meets our high quality standards.
There are 18 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 79,526 times.
Learn more...
Nitric acid is a type of potent mineral acid used to make things like fertilizers, dyes, and high explosives. The caustic, colorless liquid is typically produced on an industrial scale using highly specialized chemical processes and equipment. However, it’s also possible to make nitric acid in various purities with common laboratory materials, such as nitrate salts, sulfuric acid, and copper. Some of the substances required for this experiment are corrosive, so be sure to don the proper safety gear before handling them. It also won't hurt to brush up on your lab safety procedures so you'll be prepared in the event of an accident.
Steps
Deriving Nitric Acid from Copper
-
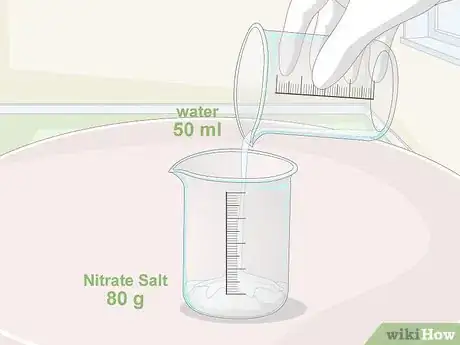
1Dissolve 80 grams (2.8 oz) of nitrate salt in 50 millilitres (1.7 fl oz) of water. Start by portioning out your nitrate salt into a small glass mixing container. Then, pour in all of the water at once. Swirl the mixture around inside the container to help it dissolve faster.[1]
- It’s important to use distilled water rather than ordinary tap water. Most tap water contains traces of other chemicals that can throw off the composition of the acid you produce.[2]
- For the purposes of this experiment, it will be easiest to use either sodium nitrate, potassium nitrate, or ammonium nitrate. All of these chemicals are readily available at any online chemistry supply store.
- Some chemistry supply stores only sell to institutions, not individuals. This may make it hard to get your hands on the items you need unless you have a teacher or supervisor order them on your behalf.
-
2Add 100 millilitres (3.4 fl oz) of hydrochloric acid. Measure the acid in a graduated cylinder to ensure that you’re using just the right amount. Transfer the acid to the nitrate salt solution carefully, taking caution not to let it spill or splash. Swirl the contents of the container again to mix your chemical components.[3]
- You can buy a bottle of concentrated hydrochloric acid for around $1 per ounce (29.57 mL).
- It’s a good idea to work under a fume hood or set up your equipment in a well-ventilated open area, as your test materials will emit strong fumes when brought into contact with the acid.
Warning: Always wear gloves and safety goggles when working with acids. Hydrochloric acid in particular is highly corrosive—without proper protection, getting even a small drop on your skin could cause serious burns.[4]
Advertisement -
3Fill a second larger container with 50 millilitres (1.7 fl oz) of water. Pour in just enough water to cover the bottom of the second container. You’ll be using this container to nest your mixing container and prevent spillage or overflow, so make sure that it’s big enough to hold your test materials comfortably.[5]
- Ideally, your second container will also be slightly taller than your mixing container.
- If you’re using a 250 mL beaker as your mixing container, you might place it in a 1,000 mL beaker in order to leave a sufficient amount of space.
-
4Place your mixing container at the bottom of the larger container. Set the mixing container down gently in the shallow water. Check to make sure that at least 1⁄4 inch (0.64 cm) of water is still exposed around all sides of the mixing container. If it’s not, find a larger container to fit it in.[6]
-
5Add at least 32 grams (1.1 oz) of copper into the acid mixture. If possible, use laboratory-grade 99% pure copper pellets. Weigh the pellets on a precision scale in a lightweight container, making sure to weigh the container itself first and subtract its weight from the final reading. When you have a minimum of 32 grams (1.1 oz), dump the copper into your mixing container.[7]
- If you can’t find copper pellets for purchase, you can also use copper chips, shavings, wire, or even coins. Keep in mind, however, that the acid mixture will mostly dissolve the copper, so you won’t get it back.[8]
- Copper is needed to kickstart the oxidation reaction that will ultimately yield nitric acid.
-
6Cover the mixing container with a separate container and wait about 1 hour. The third container will trap the nitrogen dioxide generated as the copper dissolves in the acid mixture. The gas will then flow out of the mixing container and bubble up through the water in the holding container, producing nitric acid.[9]
- A beaker that’s one or two sizes up should fit perfectly over your mixing container. You could also use a mason jar or other drinking container, provided it’s made of glass.
- It may help to rest a small, heavy object, such as a book or piece of scrap wood, over the covering container to hold it down and keep it from bobbing as the escaping gas forces it upwards.
- The nitric acid you produce with this method will only be around 40-60% pure. To make higher-purity acid, you’ll need to distill it using nitrate salts and sulfuric acid.
Distilling Pure Nitric Acid
-
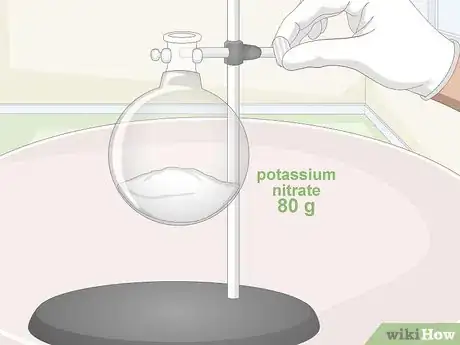
1Measure out 80 grams (2.8 oz) of pure potassium nitrate in a boiling flask. The mixture of potassium nitrate and sulfuric acid tends to produce nitric acid with the highest degree of purity. Use a precision scale to portion out an exact quantity of nitrate salt. Don’t forget to account for the weight of your measuring container.[10]
- A round-bottom boiling flask is a must for this experiment. The curved surface and tapered mouth of the flask will prevent the evaporated nitric acid from escaping during the distillation process.
- You can also use sodium or ammonium nitrate if you have them, though the purity of the resulting acid may be considerably lower.[11]
-
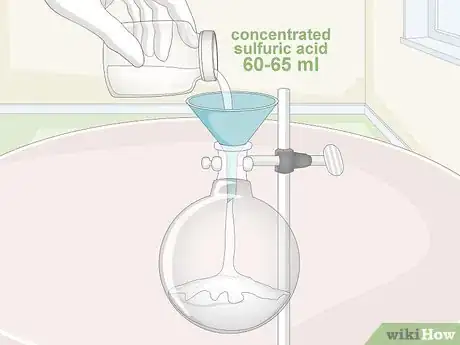
2Add 60–65 millilitres (2.0–2.2 fl oz) of concentrated sulfuric acid. Pour the acid into the boiling flask using a plastic funnel. If you don’t have a funnel handy, you can also use a dropper tool, provided it’s labelled with precise increments for liquid measurement.[12]
- Work under a fume hood or in a well-ventilated outdoor space, and be sure to put on gloves and safety goggles prior to handling any of the required chemicals.[13]
- The greater the purity of your acid, the better. Using sulfuric acid that’s been diluted with water will result in weaker nitric acid.
Tip: If you happen to come into direct contact with an acid by accident, flush the affected area with cold water for at least 15-20 minutes.
-
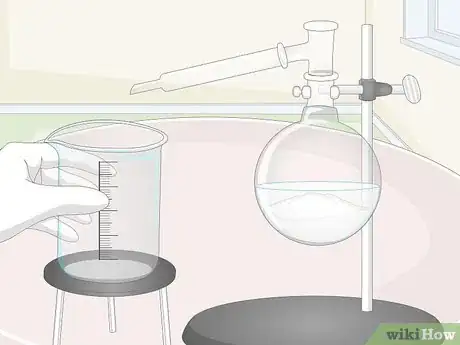
3Set up an empty glass container and a condenser for distillation. Insert a still head with a glass stopper into the mouth of the boiling flask. Connect the angled stem of the stillhead to a condenser tube. Position the lower end of the condenser over the opening of the empty container.[14]
- You can pick up a basic distillation kit, complete with everything you need to perform your own chemical distillations at home, from any chemistry supply store.
- Double-check that the stoppers on your distillation apparatus are secure. If they're not, it’s possible for harmful fumes to leak out.
-
4Heat the acid mixture for about 1 hour. Turn on your burner or hot plate, then sit back and watch the magic happen. As the acid mixture heats up, the nitrate salt and sulfuric acid will react to produce bisulfate and liquid nitric acid. The nitric acid will then be vaporized and move into the condenser, where it will cool into small droplets that will drain little-by-little into the empty container.[15]
- If you have a thermometer, fit it into the top of the stillhead and keep an eye on it while the acid mixture is on the heat source. The temperature inside the boiling flask will fluctuate somewhat, but it should ideally stay right around the boiling point of the acid, 337 °C (639 °F).[16]
- You may notice the concentrated nitric acid developing a yellowish hue as it collects. This is a normal side-effect of hydrogen dioxide contamination. The acid will still be pure enough to use for a variety of purposes.
-
5Blow air on your nitric acid to test its purity. Take a length of rubber tubing or a plastic drinking straw and place one end inside the mouth of the boiling flask. Gently blow into the other end. As you blow, watch for the acid to give off wispy, pale yellow fumes. Substantial fuming will only occur if your nitric acid has a purity greater than about 80%. Because of this unique effect, nitric acid with a high purity is sometimes referred to as “fuming nitric acid.”[17]
- Be extremely careful not to inhale the fumes. Doing so could cause severe irritation to your airways.
- If you’re wearing latex or nitrile gloves, take them off before testing your nitric acid. It may sound counterintuitive, but pure nitric acid can actually cause latex and nitrile to burst into flames![18]
Warnings
- Some of the procedures described here can be dangerous if not performed responsibly. For this reason, they’re best left to experienced chemists and lab personnel. If it’s your first time conducting a chemistry experiment, consider a simpler project, like changing the pH of water or neutralizing citric acid.⧼thumbs_response⧽
- Seek immediate medical attention if you notice any burning, swelling, itching, or irritation as a result of exposure to chemicals. If left untreated, chemical burns can lead to more serious symptoms, including shock.[19]⧼thumbs_response⧽
Things You’ll Need
Deriving Nitric Acid from Copper
- Nitrate salts
- Distilled water
- Hydrochloric acid
- Copper pellets
- Assorted laboratory glassware
- Graduated cylinder
- Precision scale
- Fume hood
- Rubber gloves
- Safety goggles
- Copper coins (optional—90% purity or higher)
Distilling Pure Nitric Acid
- Potassium nitrate
- Concentrated sulfuric acid
- Round-bottom boiling flask
- Graduated cylinder
- Precision scale
- Plastic funnel or dropper tool
- Distillation equipment
- Heat source
- Rubber tubing or plastic drinking straw
- Fume hood
- Rubber gloves
- Safety goggles
- Thermometer (optional)
References
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=31
- ↑ https://sciencing.com/distilled-good-control-science-projects-7418493.html
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=52
- ↑ https://www.geneseo.edu/sites/default/files/2021-11/Hydrochloric%20Acid%20Fisher_1.pdf
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=58
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=61
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=66
- ↑ https://www.angelo.edu/faculty/kboudrea/demos/copper_HNO3/Cu_HNO3.htm
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=72
- ↑ https://www.youtube.com/watch?v=imR0cDqSwec&feature=youtu.be&t=14
- ↑ https://sciencing.com/potassium-nitrate-reaction-experiments-5297155.html
- ↑ https://www.youtube.com/watch?v=imR0cDqSwec&feature=youtu.be&t=26
- ↑ https://ehs.stanford.edu/wp-content/uploads/Fume-Hood-Use.pdf
- ↑ http://www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/distillation
- ↑ https://www.youtube.com/watch?v=2yE7v4wkuZU&feature=youtu.be&t=348
- ↑ http://www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/distillation
- ↑ https://youtu.be/QmCdrDLyNXQ?t=328
- ↑ https://webfiles.ehs.ufl.edu/Assurance.pdf
- ↑ https://www.safetyandhealthmagazine.com/articles/15312-treating-chemical-burns