CUTL1
Cux1 (CUTL1, CDP, CDP/Cux) is a homeodomain protein that in humans is encoded by the CUX1 gene.[3][4][5][6]
Function
The protein encoded by this gene is a member of the homeodomain family of DNA binding proteins. It regulates gene expression, morphogenesis, and differentiation and it also plays a role in cell cycle progression, particularly at S-phase. Several alternatively spliced transcript variants of this gene have been described, but the full-length nature of some of these variants has not been determined, and the p200 isoform of Cux1 is processed proteolytically to smaller active isoforms, such as p110.[6] Cux1 DNA binding is stimulated by activation of the PAR2/F2RL1 cell-surface G-protein-coupled receptor in fibroblasts and breast-cancer epithelial cells to regulate Matrix metalloproteinase 10, Interleukin1-alpha, and Cyclo-oxygenase 2 (COX2) genes.[7]
Role in tumor growth
Genetic data from over 7,600 cancer patients shows that over 1% has the deactivated CUX1 which links to progression of tumor growth. Researchers from the Wellcome Trust Sanger Institute reported that the mutation of CUX1 reduces the inhibitory effects of a biological inhibitor, PIK3IP1 (phosphoinositide-3-kinase interacting protein 1), resulted in higher activity of the growth promoting enzyme, phosphoinositide 3-kinase (PI3K) which leads to tumor progression. Although CUX1 is mutated at a lower rate compared to other known gene mutations that cause cancer, this deactivated gene is found across many cancer types in this study to be the underlying cause of the disease.[8][9]
CASP
The CUX1 gene Alternatively Spliced Product was first reported in 1997.[11][lower-alpha 1] The CUX1 gene has up to 33 exons. CASP mRNA includes exons 1 through 14 and 25 through 33.[13] The human CASP protein is predicted to contain 678 amino acids, of which 400 are shared with CUTL1.[11] CASP protein is approximately 80 kD.[11] It lacks the DNA binding region of CUTL1,[11][14] but instead contains a trans-membrane domain that allows it to insert into lipid bilayers.[14] It has been localized to the Golgi apparatus.[14]
CASP has been reported to be part of a complex with Golgin 84 that tethers COPI vesicles and is important for retrograde transport in the Golgi and between the Golgi and endoplasmic reticulum.[15] The targeting of vesicles involves tethers and SNAREs.[15]
Interactions
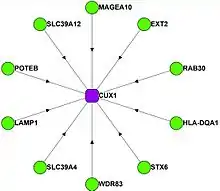
Cux1 (CUTL1, CDP, CDP/Cux) has been shown to interact with:
These physical interactions are reported in BioPlex 2.0
Notes
- This CASP is not the same as the scaffolding protein called CASP[12] for Cytohesin/ARNO ... Scaffolding Protein
References
- GRCh38: Ensembl release 89: ENSG00000257923 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Scherer SW, Neufeld EJ, Lievens PM, Orkin SH, Kim J, Tsui LC (May 1993). "Regional localization of the CCAAT displacement protein gene (CUTL1) to 7q22 by analysis of somatic cell hybrids". Genomics. 15 (3): 695–6. doi:10.1006/geno.1993.1130. PMID 8468066.
- Glöckner G, Scherer S, Schattevoy R, Boright A, Weber J, Tsui LC, Rosenthal A (December 1998). "Large-Scale Sequencing of Two Regions in Human Chromosome 7q22: Analysis of 650 kb of Genomic Sequence around the EPO and CUTL1 Loci Reveals 17 Genes". Genome Res. 8 (10): 1060–73. doi:10.1101/gr.8.10.1060. PMC 310788. PMID 9799793.
- Oka T, Ungar D, Hughson FM, Krieger M (April 2004). "The COG and COPI Complexes Interact to Control the Abundance of GEARs, a Subset of Golgi Integral Membrane Proteins". Mol. Biol. Cell. 15 (5): 2423–35. doi:10.1091/mbc.E03-09-0699. PMC 404034. PMID 15004235.
- "Entrez Gene: CUTL1 cut-like 1, CCAAT displacement protein (Drosophila)".
- Wilson BJ, Harada R, LeDuy L, Hollenberg MD, Nepveu A (January 2009). "CUX1 transcription factor is a downstream effector of the proteinase-activated receptor 2 (PAR2)". J. Biol. Chem. 284 (1): 36–45. doi:10.1074/jbc.M803808200. PMID 18952606.
- Press Release (8 December 2013). "Gene promotes one in a hundred of tumours". Wellcome Trust Sanger Institute. Retrieved 17 December 2013.
- Wong CC, Martincorena I, Rust AG, et al. (2013). "Inactivating CUX1 mutations promote tumorigenesis". Nature Genetics. 46 (1): 33–8. doi:10.1038/ng.2846. PMC 3874239. PMID 24316979.
- Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, Hirose S, Ikehara Y, Oda K (2010). "Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport". Traffic. 11 (12): 1552–66. doi:10.1111/j.1600-0854.2010.01123.x. PMID 20874812. S2CID 20424336.
- Lievens PM, Tufarelli C, Donady JJ, Stagg A, Neufeld EJ (1997). "CASP, a novel, highly conserved alternative-splicing product of the CDP/cut/cux gene, lacks cut-repeat and homeo DNA-binding domains, and interacts with full-length CDP in vitro". Gene. 197 (1–2): 73–81. doi:10.1016/s0378-1119(97)00243-6. PMID 9332351.
- Mansour M, Lee SY, Pohajdak B (2002). "The N-terminal coiled coil domain of the cytohesin/ARNO family of guanine nucleotide exchange factors interacts with the scaffolding protein CASP". The Journal of Biological Chemistry. 277 (35): 32302–9. doi:10.1074/jbc.M202898200. PMID 12052827.
- Ramdzan ZM, Nepveu A (2014). "CUX1, a haploinsufficient tumour suppressor gene overexpressed in advanced cancers". Nature Reviews Cancer. 14 (10): 673–82. doi:10.1038/nrc3805. PMID 25190083. S2CID 20468440.
- Gillingham AK, Pfeifer AC, Munro S (2002). "CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin". Molecular Biology of the Cell. 13 (11): 3761–74. doi:10.1091/mbc.E02-06-0349. PMC 133590. PMID 12429822.
- Malsam J, Satoh A, Pelletier L, Warren G (2005). "Golgin tethers define subpopulations of COPI vesicles". Science. 307 (5712): 1095–8. Bibcode:2005Sci...307.1095M. doi:10.1126/science.1108061. PMID 15718469. S2CID 12601850.
- Li S, Aufiero B, Schiltz RL, Walsh MJ (June 2000). "Regulation of the homeodomain CCAAT displacement/cut protein function by histone acetyltransferases p300/CREB-binding protein (CBP)-associated factor and CBP". Proc. Natl. Acad. Sci. U.S.A. 97 (13): 7166–71. Bibcode:2000PNAS...97.7166L. doi:10.1073/pnas.130028697. PMC 16517. PMID 10852958.
- Gupta S, Luong MX, Bleuming SA, Miele A, Luong M, Young D, Knudsen ES, Van Wijnen AJ, Stein JL, Stein GS (September 2003). "Tumor suppressor pRB functions as a co-repressor of the CCAAT displacement protein (CDP/cut) to regulate cell cycle controlled histone H4 transcription". J. Cell. Physiol. 196 (3): 541–56. doi:10.1002/jcp.10335. PMID 12891711. S2CID 2287673.
- Liu J, Barnett A, Neufeld EJ, Dudley JP (July 1999). "Homeoproteins CDP and SATB1 interact: potential for tissue-specific regulation". Mol. Cell. Biol. 19 (7): 4918–26. doi:10.1128/mcb.19.7.4918. PMC 84297. PMID 10373541.
Further reading
- Ottolenghi S, Mantovani R, Nicolis S, Ronchi A, Giglioni B (1990). "DNA sequences regulating human globin gene transcription in nondeletional hereditary persistence of fetal hemoglobin". Hemoglobin. 13 (6): 523–41. doi:10.3109/03630268908993104. PMID 2481658.
- Nepveu A (2001). "Role of the multifunctional CDP/Cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development". Gene. 270 (1–2): 1–15. doi:10.1016/S0378-1119(01)00485-1. PMID 11403998.
- Neufeld EJ, Skalnik DG, Lievens PM, Orkin SH (1993). "Human CCAAT displacement protein is homologous to the Drosophila homeoprotein, cut". Nat. Genet. 1 (1): 50–5. doi:10.1038/ng0492-50. PMID 1301999. S2CID 37107643.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Chernousov MA, Stahl RC, Carey DJ (1996). "Schwann cells secrete a novel collagen-like adhesive protein that binds N-syndecan". J. Biol. Chem. 271 (23): 13844–53. doi:10.1074/jbc.271.23.13844. PMID 8662884.
- Lievens PM, Tufarelli C, Donady JJ, Stagg A, Neufeld EJ (1997). "CASP, a novel, highly conserved alternative-splicing product of the CDP/cut/cux gene, lacks cut-repeat and homeo DNA-binding domains, and interacts with full-length CDP in vitro". Gene. 197 (1–2): 73–81. doi:10.1016/S0378-1119(97)00243-6. PMID 9332351.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Chattopadhyay S, Whitehurst CE, Chen J (1998). "A nuclear matrix attachment region upstream of the T cell receptor beta gene enhancer binds Cux/CDP and SATB1 and modulates enhancer-dependent reporter gene expression but not endogenous gene expression". J. Biol. Chem. 273 (45): 29838–46. doi:10.1074/jbc.273.45.29838. PMID 9792700.
- Liu J, Barnett A, Neufeld EJ, Dudley JP (1999). "Homeoproteins CDP and SATB1 Interact: Potential for Tissue-Specific Regulation". Mol. Cell. Biol. 19 (7): 4918–26. doi:10.1128/mcb.19.7.4918. PMC 84297. PMID 10373541.
- Rong Zeng W, Soucie E, Sung Moon N, Martin-Soudant N, Bérubé G, Leduy L, Nepveu A (2000). "Exon/intron structure and alternative transcripts of the CUTL1 gene". Gene. 241 (1): 75–85. doi:10.1016/S0378-1119(99)00465-5. PMID 10607901.
- Martin-Soudant N, Drachman JG, Kaushansky K, Nepveu A (2000). "CDP/Cut DNA binding activity is down-modulated in granulocytes, macrophages and erythrocytes but remains elevated in differentiating megakaryocytes". Leukemia. 14 (5): 863–73. doi:10.1038/sj.leu.2401764. PMID 10803519.
- Li S, Aufiero B, Schiltz RL, Walsh MJ (2000). "Regulation of the homeodomain CCAAT displacement/cut protein function by histone acetyltransferases p300/CREB-binding protein (CBP)-associated factor and CBP". Proc. Natl. Acad. Sci. U.S.A. 97 (13): 7166–71. Bibcode:2000PNAS...97.7166L. doi:10.1073/pnas.130028697. PMC 16517. PMID 10852958.
- Nirodi C, Hart J, Dhawan P, Moon NS, Nepveu A, Richmond A (2001). "The Role of CDP in the Negative Regulation of CXCL1 Gene Expression". J. Biol. Chem. 276 (28): 26122–31. doi:10.1074/jbc.M102872200. PMC 2665279. PMID 11371564.
- Moon NS, Premdas P, Truscott M, Leduy L, Bérubé G, Nepveu A (2001). "S Phase-Specific Proteolytic Cleavage Is Required To Activate Stable DNA Binding by the CDP/Cut Homeodomain Protein". Mol. Cell. Biol. 21 (18): 6332–45. doi:10.1128/MCB.21.18.6332-6345.2001. PMC 87367. PMID 11509674.
- Santaguida M, Ding Q, Bérubé G, Truscott M, Whyte P, Nepveu A (2002). "Phosphorylation of the CCAAT displacement protein (CDP)/Cux transcription factor by cyclin A-Cdk1 modulates its DNA binding activity in G(2)". J. Biol. Chem. 276 (49): 45780–90. doi:10.1074/jbc.M107978200. PMID 11584018.
- Dintilhac A, Bernués J (2002). "HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences" (PDF). J. Biol. Chem. 277 (9): 7021–8. doi:10.1074/jbc.M108417200. PMID 11748221. S2CID 39560486.
External links
- CUTL1+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human CUX1 genome location and CUX1 gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.