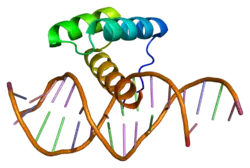
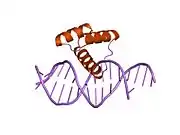
Msh homeobox 2
Homeobox protein MSX-2 is a protein that in humans is encoded by the MSX2 gene.[4][5][6]
Function
This gene encodes a member of the muscle segment homeobox gene family. The encoded protein is a transcriptional repressor whose normal activity may establish a balance between survival and apoptosis of neural crest-derived cells required for proper craniofacial morphogenesis. The encoded protein may also have a role in promoting cell growth under certain conditions and may be an important target for the RAS signaling pathways. Mutations in this gene are associated with parietal foramina 1 and craniosynostosis type 2.[6] Msx2 is a homeobox gene localized on human chromosome 5 that encodes a transcription repressor and activator (MSX-2) responsible for craniofacial and limb-bud development. Cells will express msx2 when exposed to signaling molecules BMP-2 and BMP-4 in situ.[7] Expression of msx2 leads to the proliferation, migration and osteogenic differentiation of neural crest cells during embryogenesis and bone fracture.[8] It is well documented that expression of cell-cell adhesion molecules such as E-cadherins will promote structural integrity and an epithelial arrangement of cells, while expression of N-cadherin and vimentin promote mesenchymal arrangement and cell migration.[9][10] Msx2 downregulates E-cadherins and upregulates N-cadherin and vimentin which indicates its role in inducing epithelial mesenchymal transition (EMT). Germline knockout mice have been created for this gene (Msx2 +/-) in order to examine functional loss.[11] Clinical studies on craniosynostosis, or the premature fusion of cranial structures, have shown the condition to be genetically linked to mutation in the msx2 homeobox gene.[12]
References
- GRCh38: Ensembl release 89: ENSG00000120149 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Takahashi C, Akiyama N, Matsuzaki T, Takai S, Kitayama H, Noda M (May 1996). "Characterization of a human MSX-2 cDNA and its fragment isolated as a transformation suppressor gene against v-Ki-ras oncogene". Oncogene. 12 (10): 2137–46. PMID 8668339.
- Kostrzewa M, Grady DL, Moyzis RK, Flöter L, Müller U (March 1996). "Integration of four genes, a pseudogene, thirty-one STSs, and a highly polymorphic STRP into the 7-10 Mb YAC contig of 5q34-q35". Human Genetics. 97 (3): 399–403. doi:10.1007/BF02185781. PMID 8786091. S2CID 12647370.
- "Entrez Gene: MSX2 msh homeobox 2".
- Rifas, L (July 1997). "Gestational exposure to ethanol suppresses msx2 expression in developing mouse embryos". Proc Natl Acad Sci U S A. 94 (14): 7549–54. Bibcode:1997PNAS...94.7549R. doi:10.1073/pnas.94.14.7549. PMC 23859. PMID 9207129.
- Liu H, Chen B, Li Y (March 2019). "microRNA-203 promotes proliferation, differentiation, and migration of osteoblasts by upregulation of Msh homeobox 2". Journal of Cellular Physiology. 234 (10): 17639–17648. doi:10.1002/jcp.28387. PMID 30854680. S2CID 73726197.
- Fujita T, Hayashida K, Shiba H, Kishimoto A, Matsuda S, Takeda K, Kawaguchi H, Kurihara H (August 2010). "The expressions of claudin-1 and E-cadherin in junctional epithelium". Journal of Periodontal Research. 45 (4): 579–82. doi:10.1111/j.1600-0765.2009.01258.x. PMID 20337884.
- Zhao Y, Yao J, Wu XP, Zhao L, Zhou YX, Zhang Y, You QD, Guo QL, Lu N (June 2015). "Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway". Molecular Carcinogenesis. 54 Suppl 1: E81-93. doi:10.1002/mc.22182. PMID 24976450. S2CID 29685898.
- Yu Z, Yu W, Liu J, Wu D, Wang C, Zhang J, Zhao J (July 2018). "Lens-specific deletion of the Msx2 gene increased apoptosis by enhancing the caspase-3/caspase-8 signaling pathway". The Journal of International Medical Research. 46 (7): 2843–2855. doi:10.1177/0300060518774687. PMC 6124292. PMID 29921154.
- Melville H, Wang Y, Taub PJ, Jabs EW (December 2010). "Genetic basis of potential therapeutic strategies for craniosynostosis". American Journal of Medical Genetics. Part A. 152A (12): 3007–15. doi:10.1002/ajmg.a.33703. PMID 21082653. S2CID 24424024.
- Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen MM, Abate-Shen C (May 1997). "Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism". Molecular and Cellular Biology. 17 (5): 2920–32. doi:10.1128/mcb.17.5.2920. PMC 232144. PMID 9111364.
Further reading
- Suzuki M, Tanaka M, Iwase T, Naito Y, Sugimura H, Kino I (July 1993). "Over-expression of HOX-8, the human homologue of the mouse Hox-8 homeobox gene, in human tumors". Biochemical and Biophysical Research Communications. 194 (1): 187–93. doi:10.1006/bbrc.1993.1802. hdl:10271/1007. PMID 7687426. S2CID 27890243.
- Semenza GL, Wang GL, Kundu R (April 1995). "DNA binding and transcriptional properties of wild-type and mutant forms of the homeodomain protein Msx2". Biochemical and Biophysical Research Communications. 209 (1): 257–62. doi:10.1006/bbrc.1995.1497. PMID 7726844.
- Iimura T (December 1994). "[Molecular cloning and expression of homeobox-containing genes during hard tissue development]". Kokubyo Gakkai Zasshi. The Journal of the Stomatological Society, Japan. 61 (4): 590–604. doi:10.5357/koubyou.61.590. PMID 7897272. S2CID 2781509.
- Hodgkinson JE, Davidson CL, Beresford J, Sharpe PT (July 1993). "Expression of a human homeobox-containing gene is regulated by 1,25(OH)2D3 in bone cells". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1174 (1): 11–6. doi:10.1016/0167-4781(93)90086-s. PMID 8101453.
- Jabs EW, Müller U, Li X, Ma L, Luo W, Haworth IS, Klisak I, Sparkes R, Warman ML, Mulliken JB (November 1993). "A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis". Cell. 75 (3): 443–50. doi:10.1016/0092-8674(93)90379-5. PMID 8106171. S2CID 13650758.
- Ma L, Golden S, Wu L, Maxson R (December 1996). "The molecular basis of Boston-type craniosynostosis: the Pro148-->His mutation in the N-terminal arm of the MSX2 homeodomain stabilizes DNA binding without altering nucleotide sequence preferences". Human Molecular Genetics. 5 (12): 1915–20. doi:10.1093/hmg/5.12.1915. PMID 8968743.
- Quinn LM, Johnson BV, Nicholl J, Sutherland GR, Kalionis B (March 1997). "Isolation and identification of homeobox genes from the human placenta including a novel member of the Distal-less family, DLX4". Gene. 187 (1): 55–61. doi:10.1016/S0378-1119(96)00706-8. PMID 9073066.
- Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen MM, Abate-Shen C (May 1997). "Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism". Molecular and Cellular Biology. 17 (5): 2920–32. doi:10.1128/mcb.17.5.2920. PMC 232144. PMID 9111364.
- Wu L, Wu H, Ma L, Sangiorgi F, Wu N, Bell JR, Lyons GE, Maxson R (July 1997). "Miz1, a novel zinc finger transcription factor that interacts with Msx2 and enhances its affinity for DNA". Mechanisms of Development. 65 (1–2): 3–17. doi:10.1016/S0925-4773(97)00032-4. PMID 9256341.
- Newberry EP, Latifi T, Battaile JT, Towler DA (August 1997). "Structure-function analysis of Msx2-mediated transcriptional suppression". Biochemistry. 36 (34): 10451–62. doi:10.1021/bi971008x. PMID 9265625.
- Stelnicki EJ, Kömüves LG, Holmes D, Clavin W, Harrison MR, Adzick NS, Largman C (October 1997). "The human homeobox genes MSX-1, MSX-2, and MOX-1 are differentially expressed in the dermis and epidermis in fetal and adult skin". Differentiation; Research in Biological Diversity. 62 (1): 33–41. doi:10.1046/j.1432-0436.1997.6210033.x. PMID 9373945.
- Iimura T, Takeda K, Goseki M, Maruoka Y, Sasaki S, Oida S (1998). "Characterization of two length cDNA for human MSX-2 from dental pulp-derived cells". DNA Sequence. 8 (1–2): 87–92. doi:10.3109/10425179709020891. PMID 9522127.
- Newberry EP, Latifi T, Towler DA (August 1999). "The RRM domain of MINT, a novel Msx2 binding protein, recognizes and regulates the rat osteocalcin promoter". Biochemistry. 38 (33): 10678–90. doi:10.1021/bi990967j. PMID 10451362.
- Wilkie AO, Tang Z, Elanko N, Walsh S, Twigg SR, Hurst JA, Wall SA, Chrzanowska KH, Maxson RE (April 2000). "Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification". Nature Genetics. 24 (4): 387–90. doi:10.1038/74224. PMID 10742103. S2CID 21030594.
- Wuyts W, Reardon W, Preis S, Homfray T, Rasore-Quartino A, Christians H, Willems PJ, Van Hul W (May 2000). "Identification of mutations in the MSX2 homeobox gene in families affected with foramina parietalia permagna". Human Molecular Genetics. 9 (8): 1251–5. doi:10.1093/hmg/9.8.1251. PMID 10767351.
- Quinn LM, Latham SE, Kalionis B (2000). "The homeobox genes MSX2 and MOX2 are candidates for regulating epithelial-mesenchymal cell interactions in the human placenta". Placenta. 21 Suppl A (Suppl A): S50-4. doi:10.1053/plac.1999.0514. PMID 10831122.
- Masuda Y, Sasaki A, Shibuya H, Ueno N, Ikeda K, Watanabe K (February 2001). "Dlxin-1, a novel protein that binds Dlx5 and regulates its transcriptional function". The Journal of Biological Chemistry. 276 (7): 5331–8. doi:10.1074/jbc.M008590200. PMID 11084035.
- Shirakabe K, Terasawa K, Miyama K, Shibuya H, Nishida E (October 2001). "Regulation of the activity of the transcription factor Runx2 by two homeobox proteins, Msx2 and Dlx5". Genes to Cells. 6 (10): 851–6. doi:10.1046/j.1365-2443.2001.00466.x. PMID 11683913. S2CID 22071040.
External links
- GeneReviews/NCBI/UW/NIH entry on Enlarged Parietal Foramina/Cranium Bifidum
- MSX2+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- MSX2 human gene location in the UCSC Genome Browser.
- MSX2 human gene details in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.