Rel homology domain
The Rel homology domain (RHD) is a protein domain found in a family of eukaryotic transcription factors,[2] including both NF-κB and NFAT, among others. Some of these transcription factors appear to form multi-protein DNA-bound complexes.[3] Phosphorylation of the RHD appears to play a role in the regulation of some of these transcription factors, acting to modulate the expression of their target genes.[4]
| Rel homology domain (RHD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbol | RHD | ||||||||||
| Pfam | PF00554 | ||||||||||
| InterPro | IPR011539 | ||||||||||
| PROSITE | PDOC00924 | ||||||||||
| SCOP2 | 1svc / SCOPe / SUPFAM | ||||||||||
| CDD | cd07827 | ||||||||||
| |||||||||||
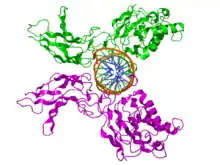
The RHD is composed of two immunoglobulin-like beta barrel subdomains that grip the DNA in the major groove. The N-terminal specificity domain resembles the core domain of the p53 transcription factor, and contains a recognition loop that interacts with DNA bases. In the case of NF-κB, the C-terminal dimerization subdomain determines dimerization propensity with other proteins in the NF-κB/Rel protein family. The dimerization subdomain is immediately followed by a nuclear localization sequence that also comprises the site for inhibitory interactions with IκB.[1]
References
- PDB: 1SVC;Müller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC (January 1995). "Structure of the NF-kappa B p50 homodimer bound to DNA". Nature. 373 (6512): 311–317. doi:10.1038/373311a0. PMID 7830764. S2CID 4285677.
- Biancalana M, Natan E, Lenardo MJ, Fersht AR (September 2021). "NF-κB Rel subunit exchange on a physiological timescale". Protein Science. 30 (9): 1818–1832. doi:10.1002/pro.4134. PMC 8376415. PMID 34089216.
- Wolberger C (October 1998). "Combinatorial transcription factors". Current Opinion in Genetics & Development. 8 (5): 552–559. doi:10.1016/S0959-437X(98)80010-5. PMID 9794820.
- Anrather J, Racchumi G, Iadecola C (January 2005). "cis-acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-kappa B". The Journal of Biological Chemistry. 280 (1): 244–252. doi:10.1074/jbc.M409344200. PMID 15516339.