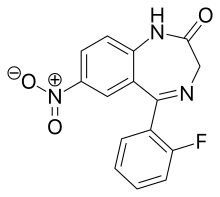
Desmethylflunitrazepam
Desmethylflunitrazepam (also known as norflunitrazepam, Ro05-4435 and fonazepam) is a benzodiazepine that is a metabolite of flunitrazepam[1][2][3] and has been sold online as a designer drug.[4][5] It has an IC50 value of 1.499 nM for the GABAA receptor.[6][7]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.018.072 |
| Chemical and physical data | |
| Formula | C15H10FN3O3 |
| Molar mass | 299.261 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Busker RW, van Henegouwen GM, Kwee BM, Winkens JH (May 1987). "Photobinding of flunitrazepam and its major photo-decomposition product N-desmethylflunitrazepam". International Journal of Pharmaceutics. 36 (2–3): 113–120. doi:10.1016/0378-5173(87)90145-1.
- Coller JK, Somogyi AA, Bochner F (November 1998). "Quantification of flunitrazepam's oxidative metabolites, 3-hydroxyflunitrazepam and desmethylflunitrazepam, in hepatic microsomal incubations by high-performance liquid chromatography". Journal of Chromatography. B, Biomedical Sciences and Applications. 719 (1–2): 87–92. doi:10.1016/S0378-4347(98)00383-1. PMID 9869368.
- Kilicarslan T, Haining RL, Rettie AE, Busto U, Tyndale RF, Sellers EM (April 2001). "Flunitrazepam metabolism by cytochrome P450S 2C19 and 3A4". Drug Metabolism and Disposition. 29 (4 Pt 1): 460–5. PMID 11259331.
- Moosmann B, Bisel P, Franz F, Huppertz LM, Auwärter V (November 2016). "Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines - an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam and nitrazolam". Journal of Mass Spectrometry. 51 (11): 1080–1089. Bibcode:2016JMSp...51.1080M. doi:10.1002/jms.3840. PMID 27535017.
- Katselou M, Papoutsis I, Nikolaou P, Spiliopoulou C, Athanaselis S (2016). "Metabolites replace the parent drug in the drug arena. The cases of fonazepam and nifoxipam". Forensic Toxicology. 35 (1): 1–10. doi:10.1007/s11419-016-0338-5. PMC 5214877. PMID 28127407.
- Maddalena DJ, Johnston GA (February 1995). "Prediction of receptor properties and binding affinity of ligands to benzodiazepine/GABAA receptors using artificial neural networks". Journal of Medicinal Chemistry. 38 (4): 715–24. doi:10.1021/jm00004a017. PMID 7861419.
- So SS, Karplus M (December 1996). "Genetic neural networks for quantitative structure-activity relationships: improvements and application of benzodiazepine affinity for benzodiazepine/GABAA receptors". Journal of Medicinal Chemistry. 39 (26): 5246–56. doi:10.1021/jm960536o. PMID 8978853.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.