Vibrio
Vibrio is a genus of Gram-negative bacteria, possessing a curved-rod (comma) shape,[1][2][3][4] several species of which can cause foodborne infection, usually associated with eating undercooked seafood. Being highly salt tolerant and unable to survive in fresh water, Vibrio spp. are commonly found in various salt water environments. Vibrio spp. are facultative anaerobes that test positive for oxidase and do not form spores.[4][5] All members of the genus are motile. They are able to have polar or lateral flagellum with or without sheaths.[4][6] Vibrio species typically possess two chromosomes, which is unusual for bacteria.[7][8] Each chromosome has a distinct and independent origin of replication,[9] and are conserved together over time in the genus.[10] Recent phylogenies have been constructed based on a suite of genes (multilocus sequence analysis).[1]
| Vibrio | |
|---|---|
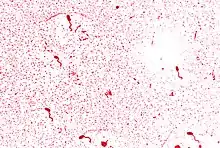 | |
| Flagellar stain of V. cholerae | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Vibrionales |
| Family: | Vibrionaceae |
| Genus: | Vibrio Pacini 1854 |
| Type species | |
| Vibrio cholerae | |
| Species | |
|
V. adaptatus | |
| Synonyms | |
| |
O. F. Müller (1773, 1786) described eight species of the genus Vibrio (included in Infusoria), three of which were spirilliforms.[11] Some of the other species are today assigned to eukaryote taxa, e.g., to the euglenoid Peranema or to the diatom Bacillaria. However, Vibrio Müller, 1773 became regarded as the name of a zoological genus, and the name of the bacterial genus became Vibrio Pacini, 1854.[12][13] Filippo Pacini isolated micro-organisms he called "vibrions" from cholera patients in 1854, because of their motility.[14] In Latin "vibrio" means "to quiver".[15]
Biochemical characteristics of Vibrio spp.
The genus Vibrio contains a large number of species. So, variation in the biochemical characteristics are most common in case of the genus Vibrio. Colony, morphological, physiological, and biochemical characteristics of the genus Vibrio are shown in the Table below.[4]
| Test type | Test | Group-1 | Group-2 |
| Colony characters | Size | Medium | Medium |
| Type | Round | Round | |
| Color | Whitish | Whitish | |
| Shape | Convex | Convex | |
| Morphological characters | Shape | Curved-rod | Curved-rod |
| Physiological characters | Motility | + | + |
| Growth at 6.5% NaCl | + | + | |
| Biochemical characters | Gram’s staining | – | – |
| Oxidase | + | + | |
| Catalase | + | + | |
| Oxidative-Fermentative | Fermentative | Oxidative | |
| Motility | + | + | |
| Methyl Red | + | – | |
| Voges-Proskauer | + | – | |
| Indole | – | – | |
| H2S Production | – | + | |
| Urease | – | + | |
| Nitrate reductase | – | + | |
| β-Galactosidase | + | + | |
| Hydrolysis of | Gelatin | + | + |
| Aesculin | – | + | |
| Casein | – | + | |
| Tween 40 | + | + | |
| Tween 60 | + | + | |
| Tween 80 | + | + | |
| Acid production from | Glycerol | + | + |
| Galactose | – | + | |
| D-Glucose | + | + | |
| D-Fructose | + | V | |
| D-Mannose | + | V | |
| Mannitol | + | V | |
| N-Acetylglucosamine | + | + | |
| Amygdalin | + | – | |
| Maltose | + | + | |
| D-Melibiose | – | – | |
| D-Trehalose | + | – | |
| Glycogen | + | + | |
| D-Turanose | + | + |
Note: Group-1: Vibrio alginolyticus; Group-2: Vibrio natriegens, Vibrio pelagius, Vibrio azureus; + = Positive; – =Negative; V =Variable (+/–)
Pathogenic strains

Several species of Vibrio are pathogens.[16] Most disease-causing strains are associated with gastroenteritis, but can also infect open wounds and cause sepsis.[17] They can be carried by numerous marine animals, such as crabs or prawns, and have been known to cause fatal infections in humans during exposure.[18] Risk of clinical disease and death increases with certain factors, such as uncontrolled diabetes, elevated iron levels (cirrhosis, sickle cell disease, hemochromatosis), and cancer or other immunocompromised states. Pathogenic Vibrio species include V. cholerae (the causative agent of cholera), V. parahaemolyticus, and V. vulnificus. V. cholerae is generally transmitted by contaminated water.[3] Pathogenic Vibrio species can cause foodborne illness (infection), usually associated with eating undercooked seafood. When ingested Vibrio bacteria can primarily result in watery diarrhea along with other secondary symptoms[19].The pathogenic features can be linked to quorum sensing, where bacteria are able to express their virulence factor via their signalling molecules.[20]
V. vulnificus outbreaks commonly occur in warm climates and small, generally lethal, outbreaks occur regularly. An outbreak occurred in New Orleans after Hurricane Katrina,[21] and several lethal cases occur most years in Florida.[22] As of 2013 in the United States, Vibrio infections as a whole were up 43% when compared with the rates observed in 2006–2008. V. vulnificus, the most severe strain, has not increased. Foodborne Vibrio infections are most often associated with eating raw shellfish.[23]
V. parahaemolyticus is also associated with the Kanagawa phenomenon, in which strains isolated from human hosts (clinical isolates) are hemolytic on blood agar plates, while those isolated from nonhuman sources are not hemolytic.[24]
Many Vibrio species are also zoonotic. They cause disease in fish and shellfish, and are common causes of mortality among domestic marine life.
Diagnosis
Cholera
A common sign of Vibrio infection is cholera. Cholera primarily presents with rapid water loss by watery diarrhea. Other symptoms include vomiting and muscle cramps.[25] Water loss can lead to dehydration which can be mild to moderate to severe. Moderate to severe dehydration requires immediate treatment. V. cholerae is the most common pathogen that causes cholera. The gold standard for detecting cholera is through cultures of stool samples or rectal swabs. Identification is then done through microscopy or by agglutination of antibodies.[25] Cultures are done in thiosulfate citrate bile-salts sucrose agar. V cholerae will form yellow colonies.[26]
Vibriosis
Vibriosis is a sign of a more severe Vibrio infection. Common causes of vibriosis include consumption of raw or undercooked seafood, primarily oysters, or wound exposure to sea water. The majority of V. parahemolyticus infections can be self-limiting and symptoms include diarrhea, nausea, headaches, fever and chills. V. vulnificus can lead to a more serious disease, particularly in wound infection which can turn into necrotizing fasciitis. V. parahaemolyticus is the most common pathogen in vibriosis, however V. vulnificus is more common in people who have certain risk factors like older age, liver disease or diabetes mellitus. Like all vibrio diagnosis, vibriosis can also be determined in stool cultures. V. parahemolyticus and V. vulnificus will form green colonies.[26]
Treatment
Medical care depends on the clinical presentation and the presence of underlying medical conditions.
Vibrio gastroenteritis
Because Vibrio gastroenteritis is self-limited in most patients, no specific medical therapy is required.[27] Patients who cannot tolerate oral fluid replacement may require intravenous fluid therapy.
Although most Vibrio species are sensitive to antibiotics such as doxycycline or ciprofloxacin, antibiotic therapy does not shorten the course of the illness or the duration of pathogen excretion. However, if the patient is ill and has a high fever or an underlying medical condition, oral antibiotic therapy with doxycycline or ciprofloxacin can be initiated.[27]
Noncholera Vibrio infections
Patients with noncholera Vibrio wound infection or sepsis are much more ill and frequently have other medical conditions. Medical therapy consists of:
- Prompt initiation of effective antibiotic therapy (doxycycline or a quinolone)
- Intensive medical therapy with aggressive fluid replacement and vasopressors for hypotension and septic shock to correct acid-base and electrolytes abnormalities that may be associated with severe sepsis
- Early fasciotomy within 24 hours after development of clinical symptoms can be life-saving in patients with necrotizing fasciitis.
- Early debridement of the infected wound has an important role in successful therapy and is especially indicated to avoid amputation of fingers, toes, or limbs.
- Expeditious and serial surgical evaluation and intervention are required because patients may deteriorate rapidly, especially those with necrotizing fasciitis or compartment syndrome.
- Reconstructive surgery, such as skin grafts, are used in the recovery phase.
Prevention
Cholera
The most effective method to prevent cholera is the improvement of water and food safety. This includes the sanitation of water, proper preparation of food and community awareness of outbreaks. Prevention has been most effective in countries where cholera is endemic.
Another method is cholera vaccines. Examples of cholera vaccines include Dukoral and Vaxchora.[28]
Vibriosis
Prevention of vibriosis is mostly effected in food processing. Food items, mostly seafood, that commonly contain vibrio organisms are regularly controlled. The water that seafood is fished or farmed from is analyzed to determine microorganism content. Food processing methods like pasteurization and high pressure are used to eliminate microorganisms and pathogens.[29]
Other strains
V. harveyi is a pathogen of several aquatic animals, and is notable as a cause of luminous vibriosis in shrimp (prawns).[30] Aliivibrio fischeri (or V. fischeri) is known for its mutualistic symbiosis with the Hawaiian bobtail squid, which is dependent on microbial luminescence.[31]
Flagella
The "typical", early-discovered Vibrio species, such as V. cholerae, have a single polar flagellum (monotrichous) with sheath. Some species, such as V. parahaemolyticus and V. alginolyticus, have both a single polar flagellum with sheath and thin flagella projecting in all directions (peritrichous), and the other species, such as V. fischeri, have tufts of polar flagella with sheath (lophotrichous).[32]
Structure
Typical bacterial flagellum structure contains three components: the basal body, the hook and the filament. Like typical bacteria, Vibrio spp, have these three components, but with increased complexity in the basal body. In addition, Vibrio spp. use five or six distinct flagellum subunits to construct the flagellar filament, rather than the single flagellin found in many other bacteria. In Vibrio spp, most have a single flagellum located on one pole of the bacterium, although some species have additional flagella in peritrichous or lophotrichous arrangements. Another difference is that the gradient used to power the flagellar motor is sodium driven rather than proton driven; this creates greater torque, and Vibrio flagella have been shown to rotate over five times faster than the H+-driven flagella of E. coli. The flagellum is also surrounded by a sheath extending from the membrane. The purpose of this sheath has yet to be determined.[33]
Effect on Virulence
Motility is very important for Vibrio spp for infection. Research has shown that a variety of Vibrios mutants that are defective in flagella synthesis or non-motile are defective in infection. Loss of motility in Vibrio has shown impaired colonization and adherence to host's intestines.[33]
Natural transformation
Natural transformation is a common bacterial adaptation for DNA transfer that employs numerous bacterial gene products.[34][35] For a recipient bacterium to bind, take up, and recombine exogenous DNA into its chromosome, it must become competent, that is, enter a special physiologic state. The DNA-uptake process of naturally competent V. cholerae involves an extended competence-induced pilus and a DNA-binding protein that acts as a ratchet and reels DNA into the periplasm.[36] Natural transformation has also been described for V. fisheri,[37] V. vulnificus[38] and V. parahaemolyticus.[39]
Small RNA
V. cholerae has been used in discoveries of many bacterial small RNAs. Using sRNA-Seq and Northern blot candidate sRNAs were identified and characterised as IGR-sRNA (intragenic region), AS-sRNAs (transcribed from the antisense strand of the open reading frame (ORF) and ORF-derived.[40] One of the candidates from this study, IGR7, was shown to be involved in carbon metabolism and later renamed MtlS RNA. Other sRNAs identified in V. cholerae through genetic screens and computational methods include Qrr RNA, Vibrio regulatory RNA of OmpA, MicX sRNA, Vibrio cholerae ToxT activated RNAs, tfoR RNA, and VqmR sRNA.
See also
References
- Thompson FL, Gevers D, Thompson CC, et al. (2005). "Phylogeny and Molecular Identification of Vibrios on the Basis of Multilocus Sequence Analysis". Applied and Environmental Microbiology. 71 (9): 5107–5115. Bibcode:2005ApEnM..71.5107T. doi:10.1128/AEM.71.9.5107-5115.2005. PMC 1214639. PMID 16151093.
- Ryan KJ; Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- Faruque SM; Nair GB, eds. (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.
- Paul, Sulav Indra; Rahman, Md. Mahbubur; Salam, Mohammad Abdus; et al. (2021-12-15). "Identification of marine sponge-associated bacteria of the Saint Martin's island of the Bay of Bengal emphasizing on the prevention of motile Aeromonas septicemia in Labeo rohita". Aquaculture. 545: 737156. doi:10.1016/j.aquaculture.2021.737156. ISSN 0044-8486.
- Madigan, Michael; Martinko, John, eds. (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 978-0-13-144329-7.
- Khan, Fazlurrahman; Tabassum, Nazia; Anand, Raksha; Kim, Young-Mog (2020-10-01). "Motility of Vibrio spp.: regulation and controlling strategies". Applied Microbiology and Biotechnology. 104 (19): 8187–8208. doi:10.1007/s00253-020-10794-7. ISSN 1432-0614. PMID 32816086. S2CID 221182959.
- Trucksis, Michele; Michalski, Jane; Deng, Ying Kang; Kaper, James B. (1998-11-24). "The Vibrio cholerae genome contains two unique circular chromosomes". Proceedings of the National Academy of Sciences. 95 (24): 14464–14469. Bibcode:1998PNAS...9514464T. doi:10.1073/pnas.95.24.14464. ISSN 0027-8424. PMC 24396. PMID 9826723.
- Okada, Kazuhisa; Iida, Tetsuya; Kita-Tsukamoto, Kumiko; Honda, Takeshi (2005-01-15). "Vibrios Commonly Possess Two Chromosomes". Journal of Bacteriology. 187 (2): 752–757. doi:10.1128/JB.187.2.752-757.2005. ISSN 0021-9193. PMC 543535. PMID 15629946.
- Rasmussen, Tue; Jensen, Rasmus Bugge; Skovgaard, Ole (2007-07-11). "The two chromosomes of Vibrio cholerae are initiated at different time points in the cell cycle". The EMBO Journal. 26 (13): 3124–3131. doi:10.1038/sj.emboj.7601747. ISSN 0261-4189. PMC 1914095. PMID 17557077.
- Kirkup, Benjamin C.; Chang, LeeAnn; Chang, Sarah; et al. (2010-01-01). "Vibrio chromosomes share common history". BMC Microbiology. 10: 137. doi:10.1186/1471-2180-10-137. ISSN 1471-2180. PMC 2875227. PMID 20459749.
- Pot, B., Gillis, M., and De Ley, J., The genus Aquaspirillum. In: Balows, A., Trüper, H.G., Dworkin, M., et al. (Eds.). The prokaryotes, 2nd ed, vol. 3. Springer-Verlag. New York. 1991
- Hugh, R. (1964). The proposed conservation of the generic name Vibrio Pacini 1854 and designation of the neotype strain of Vibrio cholerae Pacini 1854
- Hugh, R. (1964). "The Proposed Conservation of the Generic Name Vibrio Pacini 1854 and Designation of the Neotype Strain of Vibrio Cholerae Pacini 1854". International Journal of Systematic and Evolutionary Microbiology. 14 (2): 87–101. doi:10.1099/0096266X-14-2-87. S2CID 84020788.
- "Filippo Pacini".
- Stöppler, MD, Melissa. "Medical Definition of Vibrio cholerae". MedTerms Dictionary. MedicineNet. Retrieved 2021-06-03.
- C.Michael Hogan. 2010. Bacteria. Encyclopedia of Earth. eds. Sidney Draggan and C.J.Cleveland, National Council for Science and the Environment, Washington, DC Archived May 11, 2011, at the Wayback Machine
- Lee, Michelle T.; Dinh, An Q.; Nguyen, Stephanie; et al. (2019-03-28). "Late-onset Vibrio vulnificus septicemia without cirrhosis". Baylor University Medical Center Proceedings. 32 (2): 286–288. doi:10.1080/08998280.2019.1580661. ISSN 0899-8280. PMC 6541083. PMID 31191157.
- Cabanillas-Beltrán, Héctor; LLausás-Magaña, Eduardo; Romero, Ricardo; et al. (2006-12-01). "Outbreak of gastroenteritis caused by the pandemic Vibrio parahaemolyticus O3 : K6 in Mexico". FEMS Microbiology Letters. 265 (1): 76–80. doi:10.1111/j.1574-6968.2006.00475.x. ISSN 0378-1097. PMID 17107421.
- "Symptoms | Vibrio Illness (Vibriosis) | CDC". www.cdc.gov. 2021-03-02. Retrieved 2021-03-30.
- Tan, Wen-Si; Muhamad Yunos, Nina Yusrina; Tan, Pui-Wan; et al. (8 July 2014). "Characterisation of a Marine Bacterium Vibrio Brasiliensis T33 Producing N-acyl Homoserine Lactone Quorum Sensing Molecules". Sensors. 14 (7): 12104–12113. Bibcode:2014Senso..1412104T. doi:10.3390/s140712104. PMC 4168498. PMID 25006994.
- Jablecki J, Norton SA, Keller GR, et al. (2005). "Infectious Disease and Dermatologic Conditions in Evacuees and Rescue Workers After Hurricane Katrina - Multiple States, August–September, 2005". Mortality and Morbidity Weekly Report. 54: 1–4.
- Bureau of Community Environmental Health, Division of Environmental Health, Florida Department of Health (2005). "Annual Report, Florida". Food and Waterborne Illness Surveillance and Investigation: 21.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Infections from some foodborne germs increased, while others remained unchanged in 2012". Centers for Disease Control. April 18, 2013. Retrieved April 19, 2013.
- Joseph S, Colwell R, Kaper J (1982). "Vibrio parahaemolyticus and related halophilic Vibrios". Crit Rev Microbiol. 10 (1): 77–124. doi:10.3109/10408418209113506. PMID 6756788.
- John D Clemens, G Balakrish Nair, Tahmeed Ahmed, et al. (2017). Cholera. The Lancet, 390(10101). 1539-1549. ISSN 0140-6736. https://doi.org/10.1016/S0140-6736(17)30559-7.
- Baker-Austin, C., Martinez-Urtaza, J., Qadri, F., et al. (2018). Vibrio spp. infections. Nature Reviews Disease Primers, 4(1). https://doi.org/10.1038/s41572-018-0010-y
- "Noncholera Vibrio Infections - Infectious Diseases". Merck Manuals Professional Edition. Retrieved 2021-03-30.
- Hsueh, B. Y., & Waters, C. M. (2019). Combating cholera. F1000Research, 8, 589. https://doi.org/10.12688/f1000research.18093.1
- Baker-Austin, C., Martinez-Urtaza, J., Qadri, F., et al. (2018). Vibrio spp. infections. Nature Reviews Disease Primers, 4(1). https://doi.org/10.1038/s41572-018-0010-y
- Austin B, Zhang XH (2006). "Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates". Letters in Applied Microbiology. 43 (2): 119–214. doi:10.1111/j.1472-765X.2006.01989.x. PMID 16869892.
- Norsworthy AN, Visick KL (November 2013). "Gimme shelter: how Vibrio fischeri successfully navigates an animal's multiple environments". Frontiers in Microbiology. 4: 356. doi:10.3389/fmicb.2013.00356. PMC 3843225. PMID 24348467.
- George M. Garrity, ed. (2005). Bergey's manual of Systematic Bacteriology. Vol. 2 Part B (2nd ed.). Springer. pp. 496–8. ISBN 978-0-387-24144-9.
- Echazarreta MA, Klose KE. Vibrio Flagellar Synthesis. Front Cell Infect Microbiol. 2019 May 1;9:131. doi: 10.3389/fcimb.2019.00131. PMID: 31119103; PMCID: PMC6504787.
- Chen I, Dubnau D (2004). "DNA uptake during bacterial transformation". Nat. Rev. Microbiol. 2 (3): 241–9. doi:10.1038/nrmicro844. PMID 15083159. S2CID 205499369.
- Bernstein H, Bernstein C, Michod RE (2018). Sex in microbial pathogens. Infection, Genetics and Evolution volume 57, pages 8-25. https://doi.org/10.1016/j.meegid.2017.10.024
- Matthey N, Blokesch M (2016). "The DNA-Uptake Process of Naturally Competent Vibrio cholerae". Trends Microbiol. 24 (2): 98–110. doi:10.1016/j.tim.2015.10.008. PMID 26614677.
- Pollack-Berti A, Wollenberg MS, Ruby EG (2010). "Natural transformation of Vibrio fischeri requires tfoX and tfoY". Environ. Microbiol. 12 (8): 2302–11. doi:10.1111/j.1462-2920.2010.02250.x. PMC 3034104. PMID 21966921.
- Gulig PA, Tucker MS, Thiaville PC, et al. (2009). "USER friendly cloning coupled with chitin-based natural transformation enables rapid mutagenesis of Vibrio vulnificus". Appl. Environ. Microbiol. 75 (15): 4936–49. Bibcode:2009ApEnM..75.4936G. doi:10.1128/AEM.02564-08. PMC 2725515. PMID 19502446.
- Chen Y, Dai J, Morris JG, Johnson JA (2010). "Genetic analysis of the capsule polysaccharide (K antigen) and exopolysaccharide genes in pandemic Vibrio parahaemolyticus O3:K6". BMC Microbiol. 10: 274. doi:10.1186/1471-2180-10-274. PMC 2987987. PMID 21044320.
- Liu, Jane M.; Livny, Jonathan; Lawrence, Michael S.; et al. (April 2009). "Experimental discovery of sRNAs in Vibrio cholerae by direct cloning, 5S/tRNA depletion and parallel sequencing". Nucleic Acids Research. 37 (6): e46. doi:10.1093/nar/gkp080. ISSN 1362-4962. PMC 2665243. PMID 19223322.
External links
- Vibrio genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID
- Bacteriological Analytical Manual Online