Ketamine
 | |
 | |
| Names | |
|---|---|
| Trade names | Ketalar, others |
| Other names | CI-581; CL-369; CM-52372-2[1] |
IUPAC name
| |
| Clinical data | |
| Drug class | NMDA receptor antagonists; General anesthetics; Dissociative hallucinogens; Analgesics; Antidepressants |
| Addiction risk | Low–moderate[2] |
| Pregnancy category | |
| Routes of use | Any[4][5][6][7] |
| Onset of action | |
| Duration of action | |
| Defined daily dose | Not established[10] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability |
|
| Protein binding | 12–47% (low)[12][8][18] |
| Metabolism | Liver (N-demethylation):[6][19] |
| Metabolites |
|
| Elimination half-life | |
| Excretion | |
| Chemical and physical data | |
| Formula | C13H16ClNO |
| Molar mass | 237.725 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture:[8]
|
| Melting point | 258 to 261 °C (496 to 502 °F) |
SMILES
| |
InChI
| |
Ketamine is a medication mainly used for starting and maintaining anesthesia.[21] It induces a trance-like state while providing pain relief, sedation, and memory loss.[22] Other uses include sedation in intensive care and treatment of pain and depression.[23][24][16][25][26] Heart function, breathing, and airway reflexes generally remain functional.[22] Effects typically begin within five minutes when given by injection, and last up to approximately 25 minutes.[21][27]
Common side effects include agitation, confusion, or hallucinations as the medication wears off.[21][28][29] Elevated blood pressure and muscle tremors are relatively common.[21][29] Spasms of the larynx may rarely occur.[21] Ketamine is an NMDA receptor antagonist, but it may also have other actions.[30]
Ketamine was discovered in 1962, first tested in humans in 1964, and approved for use in the United States in 1970.[27][31] It was extensively used for surgical anesthesia in the Vietnam War due to its safety.[31] It is on the World Health Organization's List of Essential Medicines.[32] It is available as a generic medication.[21] The wholesale price in the developing world is between US$0.84 and US$3.22 per vial.[33] Ketamine is also used as a recreational drug for its hallucinogenic and dissociative effects.[34]
Medical uses
Anesthesia
Uses as an anesthetic:[35]
- Anesthesia in children, as the sole anesthetic for minor procedures or as an induction agent followed by neuromuscular blocker and tracheal intubation
- Asthmatics or people with chronic obstructive airway disease
- As a sedative for physically painful procedures in emergency departments[22]
- Emergency surgery in field conditions in war zones
- To supplement spinal or epidural anesthesia/analgesia using low doses
- To prevent opioid-induced hyperalgesia [36][37]
Since it suppresses breathing much less than most other available anesthetics,[38] ketamine is used in medicine as an anesthetic; however, due to the hallucinations it may cause, it is not typically used as a primary anesthetic, although it is the anesthetic of choice when reliable ventilation equipment is not available.
Ketamine is frequently used in severely injured people and appears to be safe in this group.[39] A 2011 clinical practice guideline supports the use of ketamine as a dissociative sedative in emergency medicine.[22] It is the drug of choice for people in traumatic shock who are at risk of hypotension.[40] Low blood pressure is harmful in people with severe head injury[41] and ketamine is least likely to cause low blood pressure, often even able to prevent it.[42][43]
The effect of ketamine on the respiratory and circulatory systems is different from that of other anesthetics. When used at anesthetic doses, it will usually stimulate rather than depress the circulatory system.[44] It is sometimes possible to perform ketamine anesthesia without protective measures to the airways.[45] Ketamine is considered relatively safe because protective airway reflexes are preserved.[46]
It has been successfully used to prevent postanesthetic shivering.[47]
Asthma
Ketamine is used as a bronchodilator in the treatment of severe asthma.[48] However, evidence of clinical benefit is limited.[48][49]
Seizures
Ketamine is sometimes used in the treatment of status epilepticus that has failed to adequately respond to standard treatments.[50]
Pain
Ketamine may be used for postoperative pain management. Low doses of ketamine may reduce morphine use, nausea, and vomiting after surgery.[51][52] It is especially useful in the prehospital setting, due to its effectiveness and low risk of respiratory depression.[53]
Ketamine has similar efficacy to opioids in a hospital emergency department setting for management of acute pain and for control of procedural pain.[54] If given intrathecally, its adverse cognitive effects are largely avoided at analgesic doses.[26]
It may also be used as an intravenous analgesic with opiates to manage otherwise intractable pain, particularly if this pain is neuropathic. It has the added benefit of counteracting spinal sensitization or wind-up phenomena experienced with chronic pain. At these doses, the psychotropic side effects are less apparent and well managed with benzodiazepines.[55] Ketamine is an analgesic that is most effective when used alongside a low-dose opioid; because, while it does have analgesic effects by itself, the doses required for adequate pain relief when it is used as the sole analgesic agent are considerably higher and far more likely to produce disorienting side effects.[55] A review article in 2013 concluded, "despite limitations in the breadth and depth of data available, there is evidence that ketamine may be a viable option for treatment-refractory cancer pain".[56]
Low-dose ketamine is sometimes used in the treatment of complex regional pain syndrome (CRPS).[57] A 2013 systematic review found only low-quality evidence to support the use of ketamine for CRPS.[58]
Depression
Ketamine is a rapid-acting antidepressant in depression.[16][59][60][61][62] It also may be effective in decreasing suicidal ideation, although based on lower quality evidence.[63][64][65] The antidepressant effects of ketamine were first shown in small studies in 2000 and 2006.[13] They have since been demonstrated and characterized in subsequent studies.[13] A single low, sub-anesthetic dose of ketamine given via intravenous infusion may produce antidepressant effects within four hours in people with depression.[13] These antidepressant effects may persist for up to several weeks following a single infusion.[13][66] This is in contrast to conventional antidepressants like selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs), which generally require at least several weeks for their benefits to occur and become maximal.[13] Moreover, based on the available preliminary evidence, the magnitude of the antidepressant effects of ketamine appears to be more than double that of conventional antidepressants.[13] On the basis of these findings, some have described ketamine as the single most important advance in the treatment of depression in over 50 years.[66][14] It has sparked interest in NMDA receptor antagonists for depression, and has shifted the direction of antidepressant research and development.[67]
Ketamine has not been approved for use as an antidepressant, but its enantiomer, esketamine, was developed as a nasal spray for treatment-resistant depression and was approved for this indication in the United States in March 2019.[67][13] The effectiveness of esketamine is limited however, with significant effectiveness for treatment-resistant depression seen in only two of five clinical trials.[14] Prescrire has described the harm / benefit balance in treatment resistant depression as "not acceptable" as of 2021.[68] There is also a lack of consensus on dosing and the effects and safety of long-term therapy.[62][69] Ketamine can produce euphoria and dissociative hallucinogen effects at higher doses, and thus has an abuse potential.[13][70] Moreover, ketamine has been associated with cognitive deficits, urotoxicity, hepatotoxicity, and other complications in some individuals with long-term use.[13][70] These undesirable effects may serve to limit the use of ketamine and esketamine for depression.[13][70]
Dosage
For rapid sequence intubation a dose of 1 to 3 mg per kg intravenously or 3 to 4 mg per kg injected into a muscle may be used.[71] Following intubation a dose of 1 to 6 mg per kg per hour may be used.[72]
For procedural sedation 1 to 2 mg per kg intravenously followed by additional doses of 0.5 to 1 mg per kg may be used.[72] Intramuscular injections of 4 to 5 mg / kg may also be used.[72] In young children 3 to 6 mg per kg may be sprayed in the nose.[72]
For pain management a dose of 0.1 to 0.3 mg per kg intravenously over 15 minutes is used.[72] For exited delirium a dose of 4 to 6 mg per kg by injection into a muscle or 1 mg per kg intravenously may be used.[72]
 A 1000 mg/10 mL vial of ketamine.
A 1000 mg/10 mL vial of ketamine. Two doses of intravenous ketamine, 100 mg/2 ml and 20 mg /2 ml
Two doses of intravenous ketamine, 100 mg/2 ml and 20 mg /2 ml
Side effects
When administered by medical professionals, ketamine is generally safe for those people who are critically ill.[73] Even in these cases, there are known side effects that include one or more of the following:[74]
- Heart: abnormal heart rhythms, slow heart rate or fast heart rate, high blood pressure or low blood pressure
- Central nervous system: Ketamine is traditionally avoided in people with or at risk of intracranial hypertension (ICP) due to concerns about ketamine causing increased intracranial pressure. It does not increase ICP more than opioids.[75]
- Skin: Transient reddening of the skin, transient measles-like rash
- Gastrointestinal: reduced appetite, nausea, increased salivation, vomiting
- Local: Pain, eruptions or rashes at the injection site
- Neuromuscular and skeletal: Increased skeletal muscle tone (tonic-clonic movements)
- Ocular: Double vision, increased intraocular pressure, involuntary eye movements, tunnel vision
- Respiratory: Airway obstruction, cessation of breathing, increased bronchial secretions, reduced effort to breathe, spasm of the vocal cords (larynx)
- Other: Anaphylaxis, dependence, emergence reaction
Tonic-clonic movements are reported at higher anesthetic doses in greater than 10% of people.[9]
Laryngospasm
Laryngospasm occurs in about 0.3% of people.[72] It is more common with large doses or when the medication is given by rapid intravenous push.[72] Treatment of laryngospasm due to ketamine may include: jaw thrust, bag valve mask with PEEP, further sedation with propofol, or paralysis and intubation.[72]
Cautions
The use of ketamine is cautioned against in cases of:[76][7]
- Conditions worsened by an increase in blood pressure or heart rate, such as angina, stroke, poorly controlled high blood pressure. Ketamine increases both heart rate and blood pressure.
- Psychiatric disorders: Ketamine can cause hallucinations, and therefore may exacerbate the symptoms of certain psychiatric disorders.
- Ketamine was once thought to cause increased intracranial pressure (IICP): as of 2014, this is believed not to be the case.[77]
- Raised intraocular pressure (IOP): Ketamine can further increase IOP.
- Penetrating eye injury: Can increase risk of loss of eye contents, due to increased IOP.
- Acute porphyria: Ketamine is considered porphyrinogenic, that is, it may provoke an attack of acute porphyria, a disease of the nervous system, in susceptible people.
Neurological
.jpg.webp)
At anesthetic doses, 10–20% of people experience adverse reactions that occur during emergence from anesthesia, reactions that can manifest as hallucinations and delirium.[28] These reactions may be less common in some spopulations, and when administered intramuscularly, and can occur up to 24 hours postoperatively; the chance of this occurring can be reduced by minimizing stimulation to the person during recovery and pretreating with a benzodiazepine, alongside a lower dose of ketamine.[28] People who experience severe reactions may require treatment with a small dose of a short- or ultrashort-acting barbiturate.[74]
In 1989, psychiatry professor John Olney reported ketamine caused irreversible changes, known as Olney's lesions, in two small areas of the rat brain. However, the rat brain has significant differences in metabolism from the human brain; therefore such changes may not occur in humans.[78]
The first large-scale, longitudinal study of ketamine users found current frequent (averaging 20 days/month) ketamine users had increased depression and impaired memory by several measures, including verbal, short-term memory, and visual memory. Current infrequent (averaging 3.25 days/month) ketamine users and former ketamine users were not found to differ from controls in memory, attention, and psychological well-being tests. This suggests the infrequent use of ketamine does not cause cognitive deficits, and that any deficits that might occur may be reversible when ketamine use is discontinued. However, abstinent, frequent, and infrequent users all scored higher than controls on a test of delusional symptoms.[79]
Short-term exposure of cultures of GABAergic neurons to ketamine at high concentrations led to a significant loss of differentiated cells in one study, and noncell-death-inducing concentrations of ketamine (10 μg/ml) may still initiate long-term alterations of dendritic arbor in differentiated neurons. The same study also demonstrated chronic (>24 h) administration of ketamine at concentrations as low as 0.01 μg/ml can interfere with the maintenance of dendritic arbor architecture. These results raise the possibility that chronic exposure to low, subanesthetic concentrations of ketamine, while not affecting cell survival, could still impair neuronal maintenance and development.[80][81]
More recent studies of ketamine-induced neurotoxicity have focused on primates in an attempt to use a more accurate model than rodents. One such study administered daily ketamine doses consistent with typical recreational doses (1 mg/kg IV) to adolescent cynomolgus monkeys for varying periods of time.[82] Decreased locomotor activity and indicators of increased cell death in the prefrontal cortex were detected in monkeys given daily injections for six months, but not those given daily injections for one month.[82] A study conducted on rhesus monkeys found a 24-hour intravenous infusion of ketamine caused signs of brain damage in five-day-old but not 35-day-old animals.[83]
Some neonatal experts do not recommend the use of ketamine as an anesthetic agent in human neonates because of the potential adverse effects it may have on the developing brain. These neurodegenerative changes in early development have been seen with other drugs that share the same mechanism of action of NMDA receptor antagonism as ketamine.[84]
The acute effects of ketamine cause cognitive impairment, including reductions in vigilance, verbal fluency, short-term memory, and executive function, as well as schizophrenia-like perceptual changes.[85]
Urinary tract
A 2011 review found reports of irritative urinary tract symptoms from recreational use.[86] Urinary tract symptoms have been collectively referred as "ketamine-induced ulcerative cystitis" or "ketamine-induced vesicopathy", and they include urge incontinence, decreased bladder compliance, decreased bladder volume, detrusor overactivity, and painful blood in urine. Bilateral hydronephrosis and renal papillary necrosis have also been reported in some cases.[86][87] The pathogenesis of papillary necrosis has been investigated in mice, and mononuclear inflammatory infiltration in the renal papilla resulting from ketamine dependence has been suggested as a possible mechanism.[88]
The time of onset of lower urinary tract symptoms varies depending, in part, on the severity and chronicity of ketamine use; however, it is unclear whether the severity and chronicity of ketamine use correspond linearly to the presentation of these symptoms. All reported cases where the user consumed greater than 5 g/day reported symptoms of the lower urinary tract.[86] Urinary tract symptoms appear to be most common in daily ketamine users who have used the drug recreationally for an extended period of time.[87] These symptoms have presented in only one case of medical use of ketamine. However, following dose reduction, the symptoms remitted.[87]
Management of these symptoms primarily involves ketamine cessation, for which compliance is low. Other treatments have been used, including antibiotics, NSAIDs, steroids, anticholinergics, and cystodistension.[86] Both hyaluronic acid instillation and combined pentosan polysulfate and ketamine cessation have been shown to provide relief in some people, but in the latter case, it is unclear whether relief resulted from ketamine cessation, administration of pentosan polysulfate, or both. Further follow-up is required to fully assess the efficacy of these treatments.[86]
Liver
In case reports of three people treated with esketamine for relief of chronic pain, liver enzyme abnormalities occurred following repeat treatment with ketamine infusions, with the liver enzyme values returning below the upper reference limit of normal range on cessation of the drug. The result suggests liver enzymes must be monitored during such treatment.[89]
Dependence

Ketamine's potential for dependence has been established in various operant conditioning paradigms, including conditioned place preference and self-administration; further, rats demonstrate locomotor sensitization following repeated exposure to ketamine.[87] Increased subjective feelings of 'high' have been observed in healthy human volunteers exposed to ketamine.[87] Additionally, the rapid onset of effects following smoking, insufflation, and/or intramuscular injection is thought to increase the drug's recreational use potential. The short duration of effects promotes bingeing; tolerance can develop; and withdrawal symptoms, including anxiety, shaking, and palpitations, may be present in some daily users following cessation of use.[87]
Ketamine can cause a variety of urinary tract problems that are more likely to occur with heavier and/or higher dosed use, especially in those not watching for a healthy lifestyle, according to a UK study.[91][92]
Interactions
Plasma concentrations of ketamine are increased by CYP3A4 inhibitors (e.g., diazepam) and CYP2B6 inhibitors (e.g., orphenadrine) due to inhibition of its metabolism.[9][93] CYP2B6 and CYP3A4 inducers like carbamazepine, phenobarbital, phenytoin, and rifampicin may reduce plasma levels of ketamine.[93]
Other drugs which increase blood pressure may interact with ketamine in having an additive effect on blood pressure including: stimulants, SNRI antidepressants, and MAOIs. Increase blood pressure and heart rate, palpitations, and arrhythmias may be potential effects.
Ketamine may increase the effects of other sedatives in a dose-dependent manner, including, but not limited to alcohol,[94] benzodiazepines,[95] opioids,[96] quinazolinones, phenothiazines, anticholinergics, and barbiturates.[97]
Benzodiazepines may diminish the antidepressant effects of ketamine.[93] Most conventional antidepressants can likely be combined with ketamine without diminished antidepressant effectiveness or increased side effects.[93]
Pharmacology
Pharmacodynamics
Molecular interactions
| Site | Value (μM) | Type | Action | Species | Ref |
|---|---|---|---|---|---|
| NMDA (PCP) | 0.25–0.66 0.35 | Ki IC50 | Antagonist | Human | [101][102] [101] |
| GABAA | 600–1,800 | EC50 | Agonist | Human | [100][103] |
| MOR | 26–42.1 | Ki | Agonist | Various | [6][104][103] |
| MOR2 | 12.1 700 |
Ki IC50 |
Antagonist | Human | [105] |
| DOR | 205–286 | Ki | Agonist | Human | [103] |
| KOR | 23.1–60.0 29.0 |
Ki EC50 |
Agonist | Human | [103] |
| NOP | IA | ND | ND | Human | [104] |
| σ1 | 66–140 | Ki | Agonist | Rat | [106][100][102] |
| σ2 | 26.3 | Ki | Agonist | Rat | [106][102] |
| D2 | 0.05–0.5 0.4–0.9 |
Ki EC50 | Agonist | Human | [102][107][103] |
| D2High | 0.5 1.03 | Ki EC50 | Agonist | Human Rat | [108][109] [110] |
| 5-HT2A | >10 | Ki | ND | Human | [102] |
| 5-HT2AHi | ≥15 | Ki | Agonist? | Rat | [108][111] |
| 5-HT3 | 420 97 910 |
Ki Ki IC50 |
Antagonist | Human Mouse Human |
[103] |
| M1 | 45 | Ki | Antagonist | Human | [6][112] |
| M2 | 294 | Ki | Antagonist | Human | [6][112] |
| M3 | 246 | Ki | Antagonist | Human | [6][112] |
| α7 | 20 | IC50 | Antagonist | Human | [6] |
| α4β2 | 50 | IC50 | Antagonist | Human | [6] |
| ERα | 0.345 2.31 | KD IC50 | ND | Human Human | [113] [113] |
| ChE | 494 | Ki | Inhibitor | Human | [6] |
| SERT | >10 162 126 | Ki Ki IC50 | Inhibitor | Human Rat Human | [102] [114][115] [103] |
| NET | 66.8 291 | Ki IC50 | Inhibitor | Human Human | [114][115][102] [103] |
| DAT | >10 63 >10 | Ki Ki IC50 | Inhibitor | Human Rat Human | [102] [114][115] [107] |
| PCP2 | 59.4 | Ki | ND | Human | [116] |
| VGSC | 11.5 222 1,100 |
Ki IC50 ED50 |
Antagonist | Rat Rat Human |
[103] |
| VDCC | 209 | IC50 | Inhibitor | Human | [112] |
| HCN1 | 8–16 | EC50 | Inhibitor | Mouse | [117] |
| D-serine | 0.46–0.57 | EC50 | Inhibitor | Human | [103] |
| NOS | >100 | Ki | Inhibitor | Rat | [118] |
| The smaller the value, the stronger the interaction with the site. 0.12–0.84 μM: Drowsiness, dissociative, and psychotic-like effects. 0.42–0.84 μM: Analgesia. 8.41–12.62 μM: Anaesthesia. | |||||
In vitro, ketamine acts as an antagonist of the NMDA receptor, an ionotropic glutamate receptor.[30] It binds specifically to the dizocilpine (MK-801) site of the NMDA receptor, near the channel pore, and is an uncompetitive antagonist.[102] The S(+) and R(–) stereoisomers of ketamine bind to the dizocilpine site of the NMDA receptor with different affinities, the former showing approximately 2- to 3-fold greater affinity for the receptor than the latter.[112] Ketamine may also interact with and inhibit the NMDAR via another allosteric site on the receptor.[119] Its full mechanism of action is not well-understood as of 2017.[30] Because of its complex mechanism of action, interacting with numerous receptors and subreceptor systems, it is often referred to as 'a pharmacologist's nightmare'.[120][121]
Besides NMDA receptor antagonism, other actions of ketamine under laboratory research include:[98][6][100]
- Ligand of the μ-, κ-, and δ-opioid receptors[122]
- Sigma σ1 and σ2 receptor agonist[122][123][124]
- Partial agonist of the high-affinity state of the dopamine D2 receptor[108][110]
- Ligand of the serotonin 5-HT2A receptor[108]
- Potentiator of the serotonin 5-HT3 receptor[125][126]
- Muscarinic acetylcholine receptor antagonist[6][122]
- Negative allosteric modulator of nicotinic acetylcholine receptors (e.g., α7, α4β2)[6][122]
- Ligand of estrogen receptor α (ERα)[113]
- Inhibitor of cholinesterase[6]
- Inhibitor of the reuptake of serotonin, norepinephrine, and dopamine[122]
- Ligand of the PCP site 2[116]
- Blocker of voltage-gated/dependent sodium and calcium channels[122][127]
- Blocker of HCN1 cation channels[117][128]
- Decreasing extracellular levels of D-serine, which is required for activation of the NMDA receptor complex, and increasing intracellular levels of the amino acid[103]
- Inhibitor of nitric oxide synthase[122][123]
- Indirect agonist of the AMPA receptor[129]
With a few exceptions (including interactions with the D2 and D2high receptor, nicotinic acetylcholine receptors by metabolites, and ERα) however, these actions are far weaker than ketamine's antagonism of the NMDA receptor (see the activity table to the right).[6][130] A binding study assessed ketamine at 56 sites including neurotransmitter receptors and transporters and found that it had Ki (binding affinity) values of >10,000 nM at all sites except the dizocilpine site of the NMDA receptor (Ki = 659 nM), indicating a minimum of 15-fold selectivity for the NMDA receptor over any other site assessed in this study.[102]
Although ketamine is a very weak ligand of the monoamine transporters (Ki > 60,000 nM), it has been suggested that it may interact with allosteric sites on the monoamine transporters to produce monoamine reuptake inhibition.[102] However, no functional inhibition (IC50) of the human monoamine transporters has been observed with ketamine or its metabolites at concentrations of up to 10,000 nM.[107][131] Moreover, animal studies and at least three human case reports have found no interaction between ketamine and the monoamine oxidase inhibitor (MAOI) tranylcypromine, which is of importance as the combination of a monoamine reuptake inhibitor with an MAOI can produce severe toxicity such as serotonin syndrome or hypertensive crisis.[132][133] Collectively, these findings shed doubt on the involvement of monoamine reuptake inhibition in the effects of ketamine in humans.[132][131][107][133] Ketamine has been found to increase dopaminergic neurotransmission in the brain, but instead of being due to dopamine reuptake inhibition, this may be via indirect/downstream mechanisms, namely through antagonism of the NMDA receptor.[131][107]
Active metabolites of ketamine including dehydronorketamine, hydroxynorketamine, and norketamine have been found to act as negative allosteric modulators of the α7 nicotinic acetylcholine receptor in the KXa7R1 cell line (HEK293 cells transfected with rat nicotinic acetylcholine receptor genes) with subanesthetic and nanomolar potencies (e.g., IC50 = 55 nM for dehydronorketamine), whereas ketamine itself was inactive at the same concentrations (< 1 μM).[134] These findings suggest that metabolites may contribute importantly to the pharmacodynamics of ketamine by means other than NMDA receptor antagonism.[134]
Ketamine has been found to act as a potent partial agonist of the high-affinity state of the human and rat dopamine D2 receptors in multiple studies.[108][109][110] Its apparent potency for this action is similar to that of its NMDA receptor antagonism.[108][109][110] However, there are also contradictory data, with studies finding an affinity of ketamine of >10,000 nM for the regular human and rat D2 receptors,[102][107][135] and direct interactions with the D2 receptor are controversial.[136] Moreover, whereas D2 receptor agonists like bromocriptine are able to rapidly and powerfully suppress prolactin secretion,[137] subanesthetic doses of ketamine have not been found to do this in humans and in fact have been found to dose-dependently increase prolactin levels.[138][139] Imaging studies have shown mixed results on inhibition of striatal [11C] raclopride binding by ketamine in humans, with some studies finding a significant decrease and others finding no such effect.[140] However, changes in [11C] raclopride binding may be due to changes in dopamine concentrations induced by ketamine rather than binding of ketamine to the D2 receptor.[140]
Ketamine and certain metabolites have been found to act as agonists of the ERα, with affinities in the nanomolar to low micromolar range.[113] The affinity of ketamine for the ERα was about 100-fold lower than that of estradiol.[113] Ketamine and its metabolites may bind to a site on the ERα that is distinct from that of estradiol.[113]
Effects in the brain and the body
Antagonism of the NMDA receptor is thought to be responsible for the anesthetic, amnesic, dissociative, and hallucinogenic effects of ketamine.[122] The mechanism(s) of action for the antidepressant effects of ketamine at lower doses have yet to be fully elucidated.[141] NMDA receptor antagonism results in analgesia by preventing central sensitization in dorsal horn neurons; in other words, ketamine's actions interfere with pain transmission in the spinal cord.[9] Inhibition of nitric oxide synthase lowers the production of nitric oxide – a gasotransmitter involved in pain perception, hence further contributing to analgesia.[142]
Ketamine produces measurable changes in peripheral organ systems, including the cardiovascular, gastrointestinal, and respiratory systems:[142]
- Cardiovascular: Ketamine stimulates the sympathetic nervous system, resulting in cardiovascular changes.
- Gastrointestinal: Ketamine produces nausea and vomiting in 15 to 25% of individuals at anesthetic doses.[122][109]
- Respiratory: Ketamine causes bronchodilation.[143] Several mechanisms have been hypothesized to explain this effect.[143]
The exact mechanisms of these effects are not fully understood.
Antidepressant effects
It has yet to be fully understood how ketamine mediates its robust and rapid-onset antidepressant effects.[13][144][145] In any case, it has been elucidated that acute blockade of NMDA receptors in the brain results in an activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA receptors), which in turn modulate a variety of downstream signaling pathways to influence neurotransmission in the limbic system and mediate antidepressant effects of NMDA receptor antagonists like ketamine.[13][144] Such downstream actions of this activation of AMPA receptors include upregulation of brain-derived neurotrophic factor (BDNF) and activation of its signaling receptor tropomyosin receptor kinase B (TrkB), activation of the mammalian target of rapamycin (mTOR) pathway, deactivation of glycogen synthase kinase 3 (GSK-3), and inhibition of the phosphorylation of the eukaryotic elongation factor 2 (eEF2) kinase.[13][144][146][147] In addition to blockade of the NMDA receptor, the active metabolite of ketamine hydroxynorketamine, which does not interact importantly with the NMDA receptor but nonetheless indirectly activates AMPA receptors similarly, may also or alternatively be involved in the rapid-onset antidepressant effects of ketamine.[144][145] Recent research has elucidated that an acute inhibition of the lateral habenula, a part of the brain in the limbic system that has been referred to as the "anti-reward center" (projecting to and inhibiting the mesolimbic reward pathway and modulating other limbic areas), may be involved in the antidepressant effects of ketamine.[144][148][149]
Esketamine is a more potent NMDA receptor antagonist and dissociative hallucinogen than arketamine, the other enantiomer of ketamine.[14] Because NMDA receptor antagonism was thought to underlie the antidepressant effects of ketamine, esketamine was selected for clinical development as an antidepressant.[14] However, preclinical research has since indicated that arketamine, as well as (2R,6R)-hydroxynorketamine, a metabolite of arketamine with negligible affinity for the NMDA receptor, may be more effective antidepressants than esketamine.[14] In addition, non-ketamine NMDA receptor antagonists in general are less effective antidepressants than ketamine.[14] Indeed, non-ketamine NMDA receptor antagonists, including memantine, lanicemine, rislenemdaz, rapastinel, and 4-chlorokynurenine, have thus far failed to demonstrate sufficient effectiveness for depression.[14][150] It is now thought that NMDA receptor antagonism may not be responsible for the antidepressant effects of ketamine.[14][150] For these reasons, as of 2019, arketamine and (2R,6R)-hydroxynorketamine are both entering clinical trials for the treatment of depression.[14][151]
Relationships between levels and effects
Drowsiness, dissociation, and psychosis-like effects (e.g., hallucinations, delirium) are reported in patients treated with ketamine starting at circulating concentrations of around 50 to 200 ng/mL (210–841 nM), while analgesia begins at levels of approximately 100 to 200 ng/mL (421–841 nM).[152][8] The typical intravenous antidepressant dosage of ketamine used to treat depression is low and results in maximal plasma concentrations of 70 to 200 ng/mL (294–841 nM).[69] Circulating concentrations of around 2,000 to 3,000 ng/mL (8,413–12,620 nM) are employed during anesthesia, and patients may start to awaken once levels of ketamine have decreased to about 500 to 1,000 ng/mL (2,103–4,207 nM).[152][8] There is wide variation in the peak concentrations of ketamine that have been reported in association with anesthesia in the literature, with values ranging from 2,211–3,447 ng/mL (9,300–14,500 nM) to as high as 22,370 ng/mL (94,100 nM).[104][108] Bioactive concentrations of ketamine are lower than total plasma levels due to plasma protein binding,[104] although plasma protein binding is relatively low with ketamine (approximately 12 to 47% protein-bound).[18] Concentrations of ketamine in the brain have been reported to be several-fold higher than in plasma.[108]
Pharmacokinetics
Absorption
Ketamine can be absorbed by intravenous, intramuscular, oral, and topical routes due to both its water and lipid solubilities.[142] In medical settings, ketamine is usually injected intravenously or intramuscularly.[153] The medication can be started using the oral route, or people may be changed from a subcutaneous infusion once pain is controlled. Oral ketamine is easily broken down by bile acids, and hence has a low bioavailability. Often, lozenges or "gummies" for sublingual or buccal absorption prepared by a compounding pharmacy are used to combat this issue. Some specialists stop the subcutaneous infusion when the first dose of oral ketamine is given. Others gradually reduce the infusion dose as the oral dose is increased.[154]
Bioavailability through the oral route reaches 17 to 29%; bioavailability through other routes are: 93% intramuscularly, 8 to 50% intranasally, 24 to 30% sublingually, and 11 to 30% rectally.[9][8][13][16][14] The onset of action of ketamine is seconds intravenously, 1 to 5 minutes intramuscularly, 15 to 30 minutes subcutaneously, 5 to 10 minutes via insufflation, and 15 to 30 minutes orally.[8][9] Maximal concentrations of ketamine are reached in 1 to 3 minutes intravenously, 5 to 15 minutes intramuscularly, 10 to 20 minutes intranasally, 30 minutes orally, 30 to 45 minutes rectally, and 30 to 45 minutes sublingually.[155][16][14]
Distribution
Ketamine is rapidly distributed and, due to its high lipophilicity, rapidly crosses the blood–brain barrier into the central nervous system.[156] Its distribution half-life is about 7 to 11 minutes.[156] The plasma protein binding of ketamine is relatively low at 12 to 47%.[12][8][18]
Metabolism
When administered orally, ketamine undergoes first-pass metabolism, where it is biotransformed in the liver by CYP3A4 (major), CYP2B6 (minor), and CYP2C9 (minor) isoenzymes into norketamine (through N-demethylation) and ultimately dehydronorketamine.[8] Intermediate in the biotransformation of norketamine into dehydronorketamine is the hydroxylation of norketamine into hydroxynorketamine by CYP2B6 and CYP2A6. As the major metabolite of ketamine, norketamine is one-third to one-fifth as potent as an anesthetic, and plasma levels of this metabolite are three times higher than ketamine following oral administration.[142][155] Ketamine and its metabolites are also conjugated.[20]
The duration of action of ketamine in a clinical setting is 0.5 to 2 hours intramuscularly and 4 to 6 hours orally.[9]
Elimination
Ketamine is eliminated about 90% in urine and about 1 to 5% in feces.[6] The medication is excreted mostly in the form of metabolites, with only 2 to 4% remaining unchanged.[156] Dehydronorketamine, followed by norketamine, is the most prevalent metabolite detected in urine.[157]
Chemistry
Structure

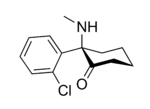
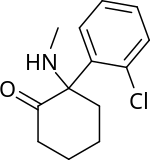
In chemical structure, ketamine is an arylcyclohexylamine derivative. Ketamine is a chiral compound. Most pharmaceutical preparations of ketamine are racemic; however, some brands reportedly have (mostly undocumented) differences in their enantiomeric proportions. The more active enantiomer, esketamine (S-ketamine), is also available for medical use under the brand name Ketanest S,[158] while the less active enantiomer, arketamine (R-ketamine), has never been marketed as an enantiopure drug for clinical use.
The optical rotation of a given enantiomer of ketamine can vary between its salts and free base form. The free base form of (S)‑ketamine exhibits dextrorotation and is therefore labelled (S)‑(+)‑ketamine. However, its hydrochloride salt shows levorotation and is thus labelled (S)‑(−)‑ketamine hydrochloride. The difference originates from the conformation of the cyclohexanone ring. In both the free base and the hydrochloride, the cyclohexanone ring adopts a chair conformation, but the orientation of the substituents varies. In the free base, the o-chlorophenyl group adopts an equatorial position and the methylamino group adopts an axial position.[159] In the hydrochloride salt, the positions are reversed, with the o-chlorophenyl group axial and the methylamino group equatorial.[160] Not all salts of ketamine show different optical rotation to the free base: (S)-ketamine (R,R)-tartrate is levorotatory, like (S)‑ketamine.[161]
| Free-base amine (equatorial chlorophenyl) |
Hydrochloride salt (axial chlorophenyl) | |
|---|---|---|
| Skeletal structure |
 |
-ketamine-hydrochloride-2D-skeletal.png.webp) |
| X-ray structure |
 |
 |
Analogues

Other arylcyclohexylamines and analogues of ketamine include eticyclidine (PCE), 3-MeO-PCE, methoxetamine (MXE), tiletamine, phencyclidine (PCP), tenocyclidine (TCP), and many others.[162]
Detection
Ketamine may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients, provide evidence in an impaired driving arrest or to assist in a medicolegal death investigation. Blood or plasma ketamine concentrations are usually in a range of 0.5–5.0 mg/L in persons receiving the drug therapeutically (during general anesthesia), 1–2 mg/L in those arrested for impaired driving and 3–20 mg/L in victims of acute fatal overdosage. Urine is often the preferred specimen for routine drug use monitoring purposes. The presence of norketamine, a pharmacologically-active metabolite, is useful for confirmation of ketamine ingestion.[163][164][165]
History
Medical use

Ketamine was first synthesized in 1962 by Calvin L. Stevens, a professor of Chemistry at Wayne State University and a Parke-Davis consultant conducting research on alpha-hydroxyimine rearrangements.[166] It was known by the developmental code name CI-581.[14] After promising preclinical research in animals, ketamine was introduced to testing in human prisoners in 1964.[31][167] These investigations demonstrated ketamine's short duration of action and reduced behavioral toxicity made it a favorable choice over phencyclidine (PCP) as a dissociative anesthetic.[168] Following FDA approval in 1970, ketamine anesthesia was first given to American soldiers during the Vietnam War.[169]
Nonmedical use
.jpg.webp)
Nonmedical use of ketamine began on the West Coast of the United States in the early 1970s.[169] Early use was documented in underground literature such as The Fabulous Furry Freak Brothers. It was used in psychiatric and other academic research through the 1970s, culminating in 1978 with the publishing of psychonaut John Lilly's The Scientist, and Marcia Moore and Howard Alltounian's Journeys into the Bright World, which documented the unusual phenomenology of ketamine intoxication.[171] The incidence of nonmedical ketamine use increased through the end of the century, especially in the context of raves and other parties.[172] However, its emergence as a club drug differs from other club drugs (e.g., MDMA) due to its anesthetic properties (e.g., slurred speech, immobilization) at higher doses;[173] in addition, there are reports of ketamine being sold as "ecstasy".[174] The use of ketamine as part of a "postclubbing experience" has also been documented.[175] Ketamine's rise in the dance culture was rapid in Hong Kong by the end of the 1990s.[173] Before becoming a federally controlled substance in the United States in 1999, ketamine was available as diverted pharmaceutical preparations and as a pure powder sold in bulk quantities from domestic chemical supply companies.[167] Much of the current ketamine diverted for nonmedical use originates in China and India.[167]
Society and culture
Generic names
Ketamine is the English generic name of the drug and its INN and BAN, while ketamine hydrochloride is its USAN, USP, BANM, and JAN.[176][177][1] Its generic name in Spanish and Italian and its DCIT are ketamina, in French and its DCF are kétamine, in German is Ketamin, and in Latin is ketaminum.[177]
The S(+) stereoisomer of ketamine is known as esketamine, and this is its BAN while esketamine hydrochloride is its BANM.[178]
Brand names
Ketamine is primarily sold throughout the world under the brand name Ketalar.[177][1] It is also marketed under a variety of other brand names, including Calypsol, Ketamin, Ketamina, Ketamine, Ketaminol, Ketanest, Ketaset, Tekam, and Vetalar among others.[177][1]
Esketamine is sold mainly under the brand names Ketanest and Ketanest-S.[178]
Clinics
After the publication of the NIH-run antidepressant clinical trial, clinics began opening in which the intravenous ketamine is given for depression.[179][180] This practice is an off label use of ketamine in the United States.[179] As of 2015 there were about 60 such clinics in the US; the procedure was not covered by insurance, and people paid between $400 and $1700 out of pocket for a treatment.[181] It was estimated in 2018 that there were approximately 300 of these clinics.[14] With the approval of esketamine for depression, it is expected that this will change.[14]
A chain of such clinics in Australia run by Aura Medical Corporation was closed down by regulatory authorities in 2015, because the clinics' marketing was not supported by scientific research and because the clinic sent people home with ketamine and needles to administer infusions to themselves.[182]
Legal status
While ketamine is legally marketed in many countries worldwide,[177] it is also a controlled substance in many countries.[6]
Australia
In Australia, ketamine is listed as a schedule 8 controlled drug under the Poisons Standard (October 2015).[183] Schedule 8 drugs are outlined in the Poisons Act 1964 as "Substances which should be available for use but require restriction of manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence."[184]
Canada
In Canada, ketamine is classified as a Schedule I narcotic, since 2005.[185]
Hong Kong
In Hong Kong, since 2000, ketamine has been regulated under Schedule 1 of Hong Kong Chapter 134 Dangerous Drugs Ordinance. It can only be used legally by health professionals, for university research purposes, or with a physician's prescription.[186][187]
Taiwan
By 2002, ketamine was classified as class III in Taiwan; given the recent rise in prevalence in East Asia, however, rescheduling into class I or II is being considered.[157][188]
India
In December 2013, the government of India, in response to rising recreational use and the use of ketamine as a date rape drug, has added it to Schedule X of the Drug and Cosmetics Act requiring a special license for sale and maintenance of records of all sales for two years.[189][190]
United Kingdom
In the United Kingdom, it became labeled a Class C drug on 1 January 2006.[157][191] On 10 December 2013, the UK Advisory Council on the Misuse of Drugs (ACMD) recommended that the government reclassify ketamine to become a Class B drug,[192] and on 12 February 2014 the Home Office announced it would follow this advice "in light of the evidence of chronic harms associated with ketamine use, including chronic bladder and other urinary tract damage".[193][194]
The UK Minister of State for Crime Prevention, Norman Baker, responding to the ACMD's advice, said the issue of its recheduling for medical and veterinary use would be addressed "separately to allow for a period of consultation".[193]
United States
The increase in recreational use prompted ketamine to be placed in Schedule III of the United States Controlled Substance Act in August 1999.[195]
Recreational use

Recreational use of ketamine was documented in the early 1970s in underground literature (e.g., The Fabulous Furry Freak Brothers).[196] It was used in psychiatric and other academic research through the 1970s, culminating in 1978 with the publishing of psychonaut John Lilly's The Scientist, and Marcia Moore and Howard Alltounian's Journeys into the Bright World, which documented the unusual phenomenology of ketamine intoxication.[197] The incidence of non-medical ketamine use increased through the end of the century, especially in the context of raves and other parties.[198][199][200][201][173] Its emergence as a club drug differs from other club drugs (e.g., MDMA), however, due to its anesthetic properties (e.g., slurred speech, immobilization) at higher doses;[173] in addition, reports of ketamine being sold as "ecstasy" are common.[202] In the 1993 book E for Ecstasy[203] (about the uses of the street drug Ecstasy in the UK), the writer, activist, and Ecstasy advocate Nicholas Saunders highlighted test results showing that certain consignments of the drug also contained ketamine. Consignments of Ecstasy known as "Strawberry" contained what Saunders described as a "potentially dangerous combination of ketamine, ephedrine, and selegiline", as did a consignment of "Sitting Duck" Ecstasy tablets.[204]
The use of ketamine as part of a "post-clubbing experience" has also been documented.[205] Ketamine's rise in the dance culture was most rapid in Hong Kong by the end of the 1990s.[173]
Ketamine use as a recreational drug has been implicated in deaths globally, with more than 90 deaths in England and Wales in the years of 2005–2013.[206] They include accidental poisonings, drownings, traffic accidents, and suicides.[206] The majority of deaths were among young people.[207] This has led to increased regulation (e.g., upgrading ketamine from a Class C to a Class B banned substance in the U.K.).[208]
Unlike the other well-known dissociatives phencyclidine (PCP) and dextromethorphan (DXM), ketamine is very short-acting. It takes effect within about 10 minutes,[209] while its hallucinogenic effects last 60 minutes when insufflated or injected and up to two hours when ingested orally.[210]
At subanesthetic doses—under-dosaged from a medical point of view—ketamine produces a dissociative state, characterised by a sense of detachment from one's physical body and the external world which is known as depersonalization and derealization.[211] At sufficiently high doses, users may experience what is called the "K-hole", a state of dissociation with visual and auditory hallucinations.[212] John C. Lilly, Marcia Moore, D. M. Turner and David Woodard (amongst others) have written extensively about their own entheogenic use of, and psychonautic experiences with, ketamine.[213] Turner died prematurely due to drowning during presumed unsupervised ketamine use.[214] In 2006 the Russian edition of Adam Parfrey's Apocalypse Culture II was banned and destroyed by authorities owing to its inclusion of an essay by Woodard about the entheogenic use of, and psychonautic experiences with, ketamine.[215]: 288–295
Because of its ability to cause confusion and amnesia, ketamine has been used for date rape.[209][169]
Slang terms
Production for recreational use has been traced to 1967, when it was referred to as "mean green" and "rockmesc".[216] Recreational names for ketamine include "Special K",[217] "K",[218][217] "Kitty", "Ket",[219] "K2",[218] "Vitamin K",[217][219] "Super K",[217] "Honey oil",[217][220] "Jet",[217][221] "Super acid",[217] "Mauve",[217] "Special LA coke",[217] "Purple",[217] "Cat Valium",[221] "Knod-off", "Skittles", "Blind Squid",[222] "Keller",[222] "Kelly's Day",[222] "New ecstasy",[223] "Psychedelic heroin",[223] "bump",[220] "Majestic".[224] A mixture of ketamine with cocaine is called "Calvin Klein" or "CK1".[225] In Hong Kong, where illicit use of the drug is popular, ketamine is colloquially referred to as "kai-jai".[173]
Usage
North America
According to the ongoing Monitoring the Future study conducted by University of Michigan, prevalence rates of recreational ketamine use among American secondary school students (grades 8, 10, and 12) have varied between 0.8–2.5% since 1999, with recent rates at the lower end of this range.[226] The 2006 National Survey on Drug Use and Health (NSDUH) reports a rate of 0.1% for persons ages 12 or older with the highest rate (0.2%) in those ages 18–25.[227] Further, 203,000 people are estimated to have used ketamine in 2006, and an estimated 2.3 million people used ketamine at least once in their life.[227] A total of 529 emergency department visits in 2009 were ketamine-related.[228]
In 2003, the U.S. Drug Enforcement Administration conducted Operation TKO, a probe into the quality of ketamine being imported from Mexico.[229] As a result of operation TKO, U.S. and Mexican authorities shut down the Mexico City company Laboratorios Ttokkyo, which was the biggest producer of ketamine in Mexico. According to the DEA, over 80% of ketamine seized in the United States is of Mexican origin. As of 2011, it was mostly shipped from places like India as cheap as $5/gram.[229] The World Health Organization Expert Committee on Drug Dependence, in its thirty-third report (2003),[230] recommended research into its recreational use due to growing concerns about its rising popularity in Europe, Asia, and North America.
Europe
Cases of ketamine use in club venues have been observed in the Czech Republic, France, Italy, Hungary, The Netherlands and the United Kingdom.[231] Additional reports of use and dependence have been reported in Poland and Portugal.[232][233]
Australia
Australia's 2010 National Drug Strategy Household Survey report shows a prevalence of recent ketamine use of 0.3% in 2004 and 0.2% in 2007 and 2010 in persons aged 14 or older.[234]
Asia
In China, the small village of Boshe in eastern Guangdong was confirmed as a main production centre when it was raided in 2013.[235]
Established by the Hong Kong Narcotics Division of the Security Bureau, the Central Registry of Drug Abuse (CRDA) maintains a database of all the illicit drug users who have come into contact with law enforcement, treatment, health care, and social organizations. The compiled data are confidential under The Dangerous Drugs Ordinance of Hong Kong, and statistics are made freely available online on a quarterly basis.[236][237] Statistics from the CRDA show that the number of ketamine users (all ages) in Hong Kong has increased from 1605 (9.8% of total drug users) in 2000 to 5212 (37.6%) in 2009.[238] Increasing trends of ketamine use among illicit drug users under the age of 21 were also reported, rising from 36.9% of young drug users in 2000 to 84.3% in 2009.[238]
A survey conducted among school-attending Taiwanese adolescents reported prevalence rates of 0.15% in 2004, 0.18% in 2005, and 0.15% in 2006 in middle-school (grades 7 and 9) students; in Taiwanese high-school (grades 10 and 12) students, prevalence was 1.13% in 2004, 0.66% in 2005, and 0.44% in 2006.[239] From the same survey, a large portion (42.8%) of those who reported ecstasy use also reported ketamine use.[239] Ketamine was the second-most used illicit drug (behind ecstasy) in absconding Taiwanese adolescents as reported by a multi-city street outreach survey.[240] In a study comparing the reporting rates between web questionnaires and paper-and-pencil questionnaires, ketamine use was reported a higher rate in the web version.[241] Urine samples taken at a club in Taipei, Taiwan showed high rates of ketamine use at 47.0%; this prevalence was compared with that of detainees suspected of recreational drug use in the general public, of which 2.0% of the samples tested positive for ketamine use.[242]
Research
Russian doctor Evgeny Krupitsky has claimed to have obtained encouraging results by using ketamine as part of a treatment for alcohol addiction which combines psychedelic and aversive techniques.[243][244] Krupitsky and Kolp summarized their work to date in 2007.[245]
Veterinary medicine
In veterinary anesthesia, ketamine is often used for its anesthetic and analgesic effects on cats,[246] dogs,[247] rabbits, rats, and other small animals.[248][249] It is highly used in induction and anesthetic maintenance in horses. It is an important part of the "rodent cocktail", a mixture of drugs used for anesthetizing rodents.[250] Veterinarians often use ketamine with sedative drugs to produce balanced anesthesia and analgesia, and as a constant-rate infusion to help prevent pain wind-up. Ketamine is used to manage pain among large animals, though it has less effect on bovines. It is the primary intravenous anesthetic agent used in equine surgery, often in conjunction with detomidine and thiopental, or sometimes guanfacine.
Ketamine appears not to produce sedation or anesthesia in snails. Instead, it appears to have an excitatory effect.[251]
References
- 1 2 3 4 Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 159–. ISBN 978-94-011-4439-1. Archived from the original on 11 April 2017.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 374–375. ISBN 978-0-07-148127-4.
Phencyclidine (PCP or angel dust) and ketamine (also known as special K) are structurally related drugs... their reinforcing properties and risks related to compulsive abuse
- 1 2 "Ketamine (Ketalar) Use During Pregnancy". Drugs.com. 22 November 2019. Archived from the original on 26 June 2020. Retrieved 18 May 2020.
- ↑ Bell RF, Eccleston C, Kalso EA (June 2017). "Ketamine as an adjuvant to opioids for cancer pain" (PDF). The Cochrane Database of Systematic Reviews. 6: CD003351. doi:10.1002/14651858.CD003351.pub3. PMC 6481583. PMID 28657160.
- ↑ Moyse DW, Kaye AD, Diaz JH, Qadri MY, Lindsay D, Pyati S (March 2017). "Perioperative Ketamine Administration for Thoracotomy Pain". Pain Physician. 20 (3): 173–184. PMID 28339431.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Mathew, Sanjay J.; Zarate Jr, Carlos A. (25 November 2016). Ketamine for Treatment-Resistant Depression: The First Decade of Progress. Springer. pp. 8–10, 14–22. ISBN 978-3-319-42925-0. Archived from the original on 8 September 2017.
- 1 2 Brayfield, A, ed. (9 January 2017). "Ketamine Hydrochloride: Martindale: The Complete Drug Reference". MedicinesComplete. London, UK: Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 24 August 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Sinner B, Graf BM (2008). "Ketamine". In Schüttler J, Schwilden H (eds.). Modern Anesthetics. Handbook of Experimental Pharmacology. Vol. 182. pp. 313–33. doi:10.1007/978-3-540-74806-9_15. ISBN 978-3-540-72813-9. PMID 18175098.
- 1 2 3 4 5 6 7 8 9 10 11 12 Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A (March 2011). "Ketamine*". Journal of Pain and Symptom Management (Therapeutic Review). 41 (3): 640–9. doi:10.1016/j.jpainsymman.2011.01.001. PMID 21419322. Archived from the original on 16 September 2018. Retrieved 9 August 2020.
- ↑ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 29 October 2020. Retrieved 2 September 2020.
- ↑ Jianren Mao (19 April 2016). Opioid-Induced Hyperalgesia. CRC Press. pp. 127–. ISBN 978-1-4200-8900-4. Archived from the original on 8 September 2017.
- 1 2 3 4 Pascal Kintz (22 March 2014). Toxicological Aspects of Drug-Facilitated Crimes. Elsevier Science. pp. 87–. ISBN 978-0-12-416969-2. Archived from the original on 8 September 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ (May 2018). "Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review". CNS Drugs. 32 (5): 411–420. doi:10.1007/s40263-018-0519-3. PMID 29736744.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Hashimoto K (October 2019). "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences. 73 (10): 613–627. doi:10.1111/pcn.12902. PMC 6851782. PMID 31215725.
- ↑ Schatzberg, Alan F.; Nemeroff, Charles B. (2017). The American Psychiatric Association Publishing Textbook of Psychopharmacology, Fifth Edition. American Psychiatric Pub. pp. 550–. ISBN 978-1-58562-523-9. Archived from the original on 8 September 2017.
- 1 2 3 4 5 6 Zhang K, Hashimoto K (January 2019). "An update on ketamine and its two enantiomers as rapid-acting antidepressants". Expert Review of Neurotherapeutics. 19 (1): 83–92. doi:10.1080/14737175.2019.1554434. PMID 30513009.
- ↑ Dickman, Andrew; Schneider, Jennifer (22 September 2016). The Syringe Driver: Continuous Subcutaneous Infusions in Palliative Care. Oxford University Press. pp. 114–. ISBN 978-0-19-873372-0. Archived from the original on 8 September 2017.
- 1 2 3 Dowd, Frank J.; Johnson, Bart; Mariotti, Angelo (3 September 2016). Pharmacology and Therapeutics for Dentistry – E-Book. Elsevier Health Sciences. pp. 235–. ISBN 978-0-323-44595-5. Archived from the original on 8 September 2017. Retrieved 9 August 2020.
- ↑ Hijazi Y, Boulieu R (July 2002). "Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes". Drug Metabolism and Disposition. 30 (7): 853–8. doi:10.1124/dmd.30.7.853. PMID 12065445. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- 1 2 Barry Levine (2003). Principles of Forensic Toxicology. American Association for Clinical Chemistry. pp. 282–. ISBN 978-1-890883-87-4. Archived from the original on 8 September 2017.
- 1 2 3 4 5 6 "Ketamine Injection". Drugs.com. Archived from the original on 10 December 2014. Retrieved 1 December 2014.
- 1 2 3 4 Green SM, Roback MG, Kennedy RM, Krauss B (May 2011). "Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update". Annals of Emergency Medicine. 57 (5): 449–61. doi:10.1016/j.annemergmed.2010.11.030. PMID 21256625.
- ↑ Zgaia AO, Irimie A, Sandesc D, Vlad C, Lisencu C, Rogobete A, Achimas-Cadariu P (2015). "The role of ketamine in the treatment of chronic cancer pain". Clujul Medical. 88 (4): 457–61. doi:10.15386/cjmed-500. PMC 4689236. PMID 26733743.
- ↑ Zapantis A, Leung S (September 2005). "Tolerance and withdrawal issues with sedation". Critical Care Nursing Clinics of North America. 17 (3): 211–23. doi:10.1016/j.ccell.2005.04.011. PMID 16115529.
- ↑ Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, et al. (March 2017). "Administration of ketamine for unipolar and bipolar depression". International Journal of Psychiatry in Clinical Practice. 21 (1): 2–12. doi:10.1080/13651501.2016.1254802. PMID 28097909.
- 1 2 Ritter JM, Flower RJ, Hendersen G, Loke YK, MacEwan D, Rang HP (2018). Rang and Dale's Pharmacology (Ninth ed.). Elsevier. p. 560. ISBN 9780702074462.
- 1 2 "Ketamine – CESAR". Center for Substance Abuse Research. University of Maryland. Archived from the original on 12 November 2013. Retrieved 26 September 2014.
- 1 2 3 Strayer RJ, Nelson LS (November 2008). "Adverse events associated with ketamine for procedural sedation in adults". The American Journal of Emergency Medicine. 26 (9): 985–1028. doi:10.1016/j.ajem.2007.12.005. PMID 19091264. Archived from the original on 8 September 2017.
- 1 2 "Ketamine Side Effects". drugs.com. Archived from the original on 10 December 2014. Retrieved 1 December 2014.
- 1 2 3 Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ (June 2017). "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience. 8 (6): 1122–1134. doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- 1 2 3 Domino EF (September 2010). "Taming the ketamine tiger. 1965". Anesthesiology. 113 (3): 678–84. doi:10.1097/ALN.0b013e3181ed09a2. PMID 20693870. Archived (PDF) from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Ketamine". Archived from the original on 23 August 2017. Retrieved 12 January 2016.
- ↑ Morgan CJ, Curran HV (January 2012). "Ketamine use: a review". Addiction. 107 (1): 27–38. doi:10.1111/j.1360-0443.2011.03576.x. PMID 21777321. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Kurdi MS, Theerth KA, Deva RS (September 2014). "Ketamine: Current applications in anesthesia, pain, and critical care". Anesthesia, Essays and Researches. 8 (3): 283–90. doi:10.4103/0259-1162.143110. PMC 4258981. PMID 25886322.
- ↑ Radvansky BM, Shah K, Parikh A, Sifonios AN, Le V, Eloy JD (2015). "Role of ketamine in acute postoperative pain management: a narrative review". BioMed Research International. 2015: 749837. doi:10.1155/2015/749837. PMC 4606413. PMID 26495312.
- ↑ Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L (2011). "A comprehensive review of opioid-induced hyperalgesia". Pain Physician. 14 (2): 145–61. PMID 21412369. Archived from the original on 28 August 2021. Retrieved 30 January 2022.
- ↑ Heshmati F, Zeinali MB, Noroozinia H, Abbacivash R, Mahoori A (December 2003). "Use of ketamine in severe status asthmaticus in intensive care unit". Iranian Journal of Allergy, Asthma, and Immunology. 2 (4): 175–80. PMID 17301376. Archived from the original on 6 October 2014.
- ↑ Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NG, Hohl CM (January 2015). "The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review". Annals of Emergency Medicine. 65 (1): 43–51.e2. doi:10.1016/j.annemergmed.2014.06.018. PMID 25064742.
- ↑ Nickson, Chris (7 August 2013). "Intubation, Hypotension and Shock". Life in the Fastlane (blog). Critical Care Compendium. Archived from the original on 9 February 2014. Retrieved 10 April 2014.
- ↑ Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L (October 2001). "Hypotension, hypoxia, and head injury: frequency, duration, and consequences". Archives of Surgery. 136 (10): 1118–23. doi:10.1001/archsurg.136.10.1118. PMID 11585502.
- ↑ Hemmingsen C, Nielsen JE (November 1991). "Intravenous ketamine for prevention of severe hypotension during spinal anaesthesia". Acta Anaesthesiologica Scandinavica. 35 (8): 755–7. doi:10.1111/j.1399-6576.1991.tb03385.x. PMID 1763596.
- ↑ Wong DH, Jenkins LC (May 1975). "The cardiovascular effects of ketamine in hypotensive states". Canadian Anaesthetists' Society Journal. 22 (3): 339–48. doi:10.1007/BF03004843. PMID 1139377.
- ↑ Adams HA (December 1997). "[S-(+)-ketamine. Circulatory interactions during total intravenous anesthesia and analgesia-sedation]" [S-(+)-ketamine. Circulatory interactions during total intravenous anesthesia and analgesia-sedation]. Der Anaesthesist (in Deutsch). 46 (12): 1081–7. doi:10.1007/s001010050510. PMID 9451493.
- ↑ Rosenbaum, Steven B.; Gupta, Vikas; Palacios, Jorge L. (2020), "Ketamine", StatPearls, StatPearls Publishing, PMID 29262083, archived from the original on 12 November 2020, retrieved 5 March 2020
- ↑ Wong JJ, Lee JH, Turner DA, Rehder KJ (August 2014). "A review of the use of adjunctive therapies in severe acute asthma exacerbation in critically ill children". Expert Review of Respiratory Medicine. 8 (4): 423–41. doi:10.1586/17476348.2014.915752. PMID 24993063.
- ↑ Zhou Y, Mannan A, Han Y, Liu H, Guan HL, Gao X, et al. (December 2019). "Efficacy and safety of prophylactic use of ketamine for prevention of postanesthetic shivering: a systematic review and meta analysis". BMC Anesthesiology. 19 (1): 245. doi:10.1186/s12871-019-0910-8. PMC 6937868. PMID 31888509.
- 1 2 Goyal S, Agrawal A (May 2013). "Ketamine in status asthmaticus: A review". Indian Journal of Critical Care Medicine. 17 (3): 154–61. doi:10.4103/0972-5229.117048. PMC 3777369. PMID 24082612.
- ↑ Jat KR, Chawla D (November 2012). "Ketamine for management of acute exacerbations of asthma in children". Airways Group. The Cochrane Database of Systematic Reviews. 11 (11): CD009293. doi:10.1002/14651858.CD009293.pub2. PMC 6483733. PMID 23152273.
{{cite journal}}: Unknown parameter|nopp=ignored (help) - ↑ Gomes D, Pimentel J, Bentes C, Aguiar de Sousa D, Antunes AP, Alvarez A, Silva ZC (October 2018). "Consensus Protocol for the Treatment of Super-Refractory Status Epilepticus". Acta Medica Portuguesa. 31 (10): 598–605. doi:10.20344/amp.9679. PMID 30387431. Archived from the original on 29 August 2020. Retrieved 9 August 2020.
- ↑ Bell RF, Dahl JB, Moore RA, Kalso EA (July 2015). "WITHDRAWN: Perioperative ketamine for acute postoperative pain". The Cochrane Database of Systematic Reviews (7): CD004603. doi:10.1002/14651858.cd004603.pub3. PMID 26133677.
- ↑ Sin B, Ternas T, Motov SM (March 2015). "The use of subdissociative-dose ketamine for acute pain in the emergency department". Academic Emergency Medicine. 22 (3): 251–7. doi:10.1111/acem.12604. PMID 25716117. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Svenson J, Biedermann M (2011). "Ketamine: a unique drug with several potential uses in the prehospital setting". Journal of Paramedic Practice. 3 (10): 552–556. doi:10.12968/jpar.2011.3.10.552. ISSN 1759-1376.
- ↑ Karlow N, Schlaepfer CH, Stoll CR, Doering M, Carpenter CR, Colditz GA, et al. (October 2018). "A Systematic Review and Meta-analysis of Ketamine as an Alternative to Opioids for Acute Pain in the Emergency Department". Academic Emergency Medicine. 25 (10): 1086–1097. doi:10.1111/acem.13502. PMID 30019434.
- 1 2 Elia N, Tramèr MR (January 2005). "Ketamine and postoperative pain--a quantitative systematic review of randomised trials". Pain. 113 (1–2): 61–70. doi:10.1016/j.pain.2004.09.036. PMID 15621365.
- ↑ Bredlau AL, Thakur R, Korones DN, Dworkin RH (October 2013). "Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature". Pain Medicine. 14 (10): 1505–17. doi:10.1111/pme.12182. PMID 23915253.
- ↑ Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE (September 2004). "Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome". Pain Medicine. 5 (3): 263–75. doi:10.1111/j.1526-4637.2004.04043.x. PMID 15367304.
- ↑ O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL (April 2013). "Interventions for treating pain and disability in adults with complex regional pain syndrome". Pain, Palliative and Supportive Care Group. The Cochrane Database of Systematic Reviews. 4 (4): CD009416. doi:10.1002/14651858.CD009416.pub2. PMC 6469537. PMID 23633371.
{{cite journal}}: Unknown parameter|nopp=ignored (help) - ↑ McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, et al. (September 2015). "Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults". The Cochrane Database of Systematic Reviews (9): CD011611. doi:10.1002/14651858.CD011611.pub2. PMID 26415966.
ketamine (administered intravenously) proved to be more efficacious than placebo, though the quality of evidence was limited by risk of bias and small sample sizes.
- ↑ Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015). "Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics". Annual Review of Medicine. 66: 509–23. doi:10.1146/annurev-med-053013-062946. PMC 4428310. PMID 25341010.
- ↑ Serafini G, Howland RH, Rovedi F, Girardi P, Amore M (September 2014). "The role of ketamine in treatment-resistant depression: a systematic review". Current Neuropharmacology. 12 (5): 444–61. doi:10.2174/1570159X12666140619204251. PMC 4243034. PMID 25426012.
- 1 2 Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK (April 2014). "Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy". Therapeutic Advances in Psychopharmacology. 4 (2): 75–99. doi:10.1177/2045125313507739. PMC 3952483. PMID 24688759.
- ↑ Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G (June 2017). "Ketamine as a rapid-acting agent for suicidal ideation: A meta-analysis". Neuroscience and Biobehavioral Reviews. 77: 232–236. doi:10.1016/j.neubiorev.2017.03.010. PMID 28342764.
- ↑ Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. (February 2018). "The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis". The American Journal of Psychiatry. 175 (2): 150–158. doi:10.1176/appi.ajp.2017.17040472. PMC 5794524. PMID 28969441.
- ↑ Sathyanarayana Rao TS, Andrade C (2017). "A possible role for ketamine in suicide prevention in emergency and mainstream psychiatry". Indian Journal of Psychiatry. 59 (3): 259–261. doi:10.4103/psychiatry.IndianJPsychiatry_345_17. PMC 5659073. PMID 29085082.
- 1 2 Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R (May 2017). "Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight". The Lancet. Psychiatry. 4 (5): 419–426. doi:10.1016/S2215-0366(17)30102-5. hdl:10871/30208. PMID 28395988. Archived from the original on 9 March 2019. Retrieved 9 August 2020.
- 1 2 Dhir A (January 2017). "Investigational drugs for treating major depressive disorder". Expert Opinion on Investigational Drugs. 26 (1): 9–24. doi:10.1080/13543784.2017.1267727. PMID 27960559.
- ↑ "Treatment-resistant depression: avoid esketamine (Spravato°)". english.prescrire.org. Archived from the original on 24 January 2021. Retrieved 1 February 2021.
- 1 2 Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al. (April 2017). "A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders". JAMA Psychiatry. 74 (4): 399–405. doi:10.1001/jamapsychiatry.2017.0080. PMID 28249076.
- 1 2 3 Short B, Fong J, Galvez V, Shelker W, Loo CK (January 2018). "Side-effects associated with ketamine use in depression: a systematic review". The Lancet. Psychiatry. 5 (1): 65–78. doi:10.1016/S2215-0366(17)30272-9. PMID 28757132.
- ↑ "Rapid sequence intubation - WikEM". wikem.org. Archived from the original on 3 August 2020. Retrieved 19 August 2020.
- 1 2 3 4 5 6 7 8 9 "Ketamine - WikEM". wikem.org. Archived from the original on 26 June 2020. Retrieved 19 August 2020.
- ↑ Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NG, Hohl CM (January 2015). "The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review". Annals of Emergency Medicine. 65 (1): 43–51.e2. doi:10.1016/j.annemergmed.2014.06.018. PMID 25064742.
- 1 2 Merck Manual; Drug Information Provided by Lexi-Comp. Last full review/revision May 2014 Ketamine Archived 9 March 2011 at the Wayback Machine
- ↑ Wang X, Ding X, Tong Y, Zong J, Zhao X, Ren H, Li Q (December 2014). "Ketamine does not increase intracranial pressure compared with opioids: meta-analysis of randomized controlled trials". Journal of Anesthesia. 28 (6): 821–7. doi:10.1007/s00540-014-1845-3. PMID 24859931.
- ↑ Rossi, S, ed. (July 2017). "Ketamine – Australian Medicines Handbook". AMH Online. Adelaide, Australia: Australian Medicines Handbook Pty Ltd. Archived from the original on 28 August 2021. Retrieved 24 August 2017.
- ↑ Zeiler FA, Teitelbaum J, West M, Gillman LM (August 2014). "The ketamine effect on ICP in traumatic brain injury". Neurocritical Care. 21 (1): 163–73. doi:10.1007/s12028-013-9950-y. PMID 24515638.
- ↑ Neurological effects of ketamine introduction references:
- Olney JW, Labruyere J, Price MT (June 1989). "Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs". Science. 244 (4910): 1360–2. Bibcode:1989Sci...244.1360O. doi:10.1126/science.2660263. PMID 2660263.
- Anderson, Cliff (June 2003). "The Bad News Isn't In: A Look at Evidence for Specific Mechanisms of Dissociative-Induced Brain Damage and Cognitive Impairment". Erowid. Archived from the original on 17 December 2008.
- Tryba M, Gehling M (October 2002). "Clonidine--a potent analgesic adjuvant". Current Opinion in Anaesthesiology. 15 (5): 511–7. doi:10.1097/00001503-200210000-00007. PMID 17019247.
- Dong C, Anand KJ (June 2013). "Developmental neurotoxicity of ketamine in pediatric clinical use". Toxicology Letters. 220 (1): 53–60. doi:10.1016/j.toxlet.2013.03.030. PMID 23566897.
- ↑ Morgan CJ, Muetzelfeldt L, Curran HV (January 2010). "Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study". Addiction. 105 (1): 121–33. doi:10.1111/j.1360-0443.2009.02761.x. PMID 19919593.
- ↑ Vutskits L, Gascon E, Potter G, Tassonyi E, Kiss JZ (May 2007). "Low concentrations of ketamine initiate dendritic atrophy of differentiated GABAergic neurons in culture". Toxicology. 234 (3): 216–26. doi:10.1016/j.tox.2007.03.004. PMID 17418473.
- ↑ Hargreaves RJ, Hill RG, Iversen LL (1994). Ito U, et al. (eds.). "Neuroprotective NMDA antagonists: the controversy over their potential for adverse effects on cortical neuronal morphology". Acta Neurochirurgica. Supplementum. Acta Neurochirurgica. 60: 15–9. doi:10.1007/978-3-7091-9334-1_4. ISBN 978-3-7091-9336-5. PMID 7976530.
- 1 2 Sun L, Li Q, Li Q, Zhang Y, Liu D, Jiang H, et al. (March 2014). "Chronic ketamine exposure induces permanent impairment of brain functions in adolescent cynomolgus monkeys". Addiction Biology. 19 (2): 185–94. doi:10.1111/adb.12004. PMID 23145560.
- ↑ Slikker W, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, et al. (July 2007). "Ketamine-induced neuronal cell death in the perinatal rhesus monkey". Toxicological Sciences. 98 (1): 145–58. doi:10.1093/toxsci/kfm084. PMID 17426105. Archived from the original on 8 April 2020. Retrieved 9 August 2020.
- ↑ Patel P, Sun L (April 2009). "Update on neonatal anesthetic neurotoxicity: insight into molecular mechanisms and relevance to humans". Anesthesiology (commentary). 110 (4): 703–8. doi:10.1097/ALN.0b013e31819c42a4. PMC 2737718. PMID 19276968.
- ↑ Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. (March 1994). "Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses". Archives of General Psychiatry. 51 (3): 199–214. doi:10.1001/archpsyc.1994.03950030035004. PMID 8122957.
- 1 2 3 4 5 Middela S, Pearce I (January 2011). "Ketamine-induced vesicopathy: a literature review". International Journal of Clinical Practice. 65 (1): 27–30. doi:10.1111/j.1742-1241.2010.02502.x. PMID 21155941. Archived from the original on 19 September 2018. Retrieved 9 August 2020.
- 1 2 3 4 5 6 Morgan CJ, Curran HV (January 2012). "Ketamine use: a review". Addiction. 107 (1): 27–38. doi:10.1111/j.1360-0443.2011.03576.x. PMID 21777321. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Yeung LY, Rudd JA, Lam WP, Mak YT, Yew DT (December 2009). "Mice are prone to kidney pathology after prolonged ketamine addiction". Toxicology Letters. 191 (2–3): 275–8. doi:10.1016/j.toxlet.2009.09.006. PMID 19766175.
- ↑ Bell RF (June 2012). "Ketamine for chronic noncancer pain: concerns regarding toxicity". Current Opinion in Supportive and Palliative Care. 6 (2): 183–7. doi:10.1097/SPC.0b013e328352812c. PMID 22436323. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Nutt D, King LA, Saulsbury W, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
- ↑ "Club Drug Ketamine Linked to Urinary and Bladder Problems". 5 April 2012. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ "Doctors' warning on ketamine risk". 23 November 2009. Archived from the original on 6 October 2014. Retrieved 9 August 2020.
- 1 2 3 4 Andrade C (July 2017). "Ketamine for Depression, 5: Potential Pharmacokinetic and Pharmacodynamic Drug Interactions". The Journal of Clinical Psychiatry. 78 (7): e858–e861. doi:10.4088/JCP.17f11802. PMID 28858450.
- ↑ Hui TW, Short TG, Hong W, Suen T, Gin T, Plummer J (March 1995). "Additive interactions between propofol and ketamine when used for anesthesia induction in female patients". Anesthesiology. 82 (3): 641–8. doi:10.1097/00000542-199503000-00005. PMID 7879932.
- ↑ Hong W, Short TG, Hui TW (December 1993). "Hypnotic and anesthetic interactions between ketamine and midazolam in female patients". Anesthesiology. 79 (6): 1227–32. doi:10.1097/00000542-199312000-00013. PMID 8267198.
- ↑ Akhavanakbari G, Mohamadian A, Entezariasl M (April 2014). "Evaluation the effects of adding ketamine to morphine in intravenous patient-controlled analgesia after orthopedic surgery". Perspectives in Clinical Research. 5 (2): 85–7. doi:10.4103/2229-3485.128028. PMC 3980550. PMID 24741486.
- ↑ Eker HE, Yalcin Cok O, Aribogan A, Arslan G (October 2011). "Children on phenobarbital monotherapy requires more sedatives during MRI". Paediatric Anaesthesia. 21 (10): 998–1002. doi:10.1111/j.1460-9592.2011.03606.x. PMID 21564387. Archived (PDF) from the original on 28 August 2021. Retrieved 9 August 2020.
- 1 2 Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 September 2017. Retrieved 14 August 2017.
- ↑ Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. (July 2018). "Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms". Pharmacological Reviews. 70 (3): 621–660. doi:10.1124/pr.117.015198. PMC 6020109. PMID 29945898.
- 1 2 3 4 Frohlich J, Van Horn JD (April 2014). "Reviewing the ketamine model for schizophrenia". Journal of Psychopharmacology. 28 (4): 287–302. doi:10.1177/0269881113512909. PMC 4133098. PMID 24257811.
- 1 2 Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA, Thomas CJ (September 2017). "Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites". Organic Letters. 19 (17): 4572–4575. doi:10.1021/acs.orglett.7b02177. PMC 5641405. PMID 28829612.
- 1 2 3 4 5 6 7 8 9 10 11 12 Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, Iversen L (2013). "The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor". PloS One. 8 (3): e59334. Bibcode:2013PLoSO...859334R. doi:10.1371/journal.pone.0059334. PMC 3602154. PMID 23527166.
- 1 2 3 4 5 6 7 8 9 10 11 Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. (July 2018). "Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms". Pharmacological Reviews. 70 (3): 621–660. doi:10.1124/pr.117.015198. PMC 6020109. PMID 29945898.
- 1 2 3 4 Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG (January 1999). "Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells". Anesthesiology. 90 (1): 174–82. doi:10.1097/00000542-199901000-00023. PMID 9915326.
- ↑ Hirota K, Sikand KS, Lambert DG (1999). "Interaction of ketamine with mu2 opioid receptors in SH-SY5Y human neuroblastoma cells". Journal of Anesthesia. 13 (2): 107–9. doi:10.1007/s005400050035. PMID 14530949.
- 1 2 Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR (April 2012). "Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo". European Neuropsychopharmacology. 22 (4): 308–17. doi:10.1016/j.euroneuro.2011.08.002. PMID 21911285.
- 1 2 3 4 5 6 Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, et al. (October 2016). "Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters". The Journal of Pharmacology and Experimental Therapeutics. 359 (1): 159–70. doi:10.1124/jpet.116.235838. PMC 5034706. PMID 27469513.
- 1 2 3 4 5 6 7 8 Kapur S, Seeman P (2002). "NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia". Molecular Psychiatry. 7 (8): 837–44. doi:10.1038/sj.mp.4001093. PMID 12232776.
- 1 2 3 4 Seeman P, Guan HC (November 2008). "Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia". Synapse. 62 (11): 819–28. doi:10.1002/syn.20561. PMID 18720422.
- 1 2 3 4 Seeman P, Guan HC, Hirbec H (August 2009). "Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil". Synapse. 63 (8): 698–704. doi:10.1002/syn.20647. PMID 19391150. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Rabin RA, Doat M, Winter JC (December 2000). "Role of serotonergic 5-HT2A receptors in the psychotomimetic actions of phencyclidine". The International Journal of Neuropsychopharmacology. 3 (4): 333–338. doi:10.1017/S1461145700002091. PMID 11343613.
- 1 2 3 4 5 Hirota K, Lambert DG (October 1996). "Ketamine: its mechanism(s) of action and unusual clinical uses". British Journal of Anaesthesia. 77 (4): 441–4. doi:10.1093/bja/77.4.441. PMID 8942324. Archived from the original on 20 October 2015.
- 1 2 3 4 5 6 Ho MF, Correia C, Ingle JN, Kaddurah-Daouk R, Wang L, Kaufmann SH, Weinshilboum RM (June 2018). "Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression". Biochemical Pharmacology. 152: 279–292. doi:10.1016/j.bcp.2018.03.032. PMC 5960634. PMID 29621538.
- 1 2 3 Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M (March 1998). "Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells". Anesthesiology. 88 (3): 768–74. doi:10.1097/00000542-199803000-00029. PMID 9523822.
- 1 2 3 Nishimura M, Sato K (October 1999). "Ketamine stereoselectively inhibits rat dopamine transporter". Neuroscience Letters. 274 (2): 131–4. doi:10.1016/s0304-3940(99)00688-6. PMID 10553955.
- 1 2 Rothman RB (1994). "PCP site 2: a high affinity MK-801-insensitive phencyclidine binding site". Neurotoxicology and Teratology. 16 (4): 343–53. doi:10.1016/0892-0362(94)90022-1. PMID 7968938. Archived from the original on 9 October 2020. Retrieved 9 August 2020.
- 1 2 Chen X, Shu S, Bayliss DA (January 2009). "HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine". The Journal of Neuroscience. 29 (3): 600–9. doi:10.1523/JNEUROSCI.3481-08.2009. PMC 2744993. PMID 19158287.
- ↑ Li CY, Chou TC, Wong CS, Ho ST, Wu CC, Yen MH, Ding YA (September 1997). "Ketamine inhibits nitric oxide synthase in lipopolysaccharide-treated rat alveolar macrophages". Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie. 44 (9): 989–95. doi:10.1007/BF03011971. PMID 9305563.
- ↑ Orser BA, Pennefather PS, MacDonald JF (April 1997). "Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors". Anesthesiology. 86 (4): 903–17. doi:10.1097/00000542-199704000-00021. PMID 9105235.
- ↑ Schmoldt A, Benthe HF, Haberland G (September 1975). "Digitoxin metabolism by rat liver microsomes". Biochemical Pharmacology. 24 (17): 1639–41. doi:10.1016/0006-2952(75)90094-5. PMC 5922622. PMID 10. Archived from the original on 4 March 2021. Retrieved 30 January 2022.
- ↑ Potter DE, Choudhury M (December 2014). "Ketamine: repurposing and redefining a multifaceted drug". Drug Discovery Today. 19 (12): 1848–54. doi:10.1016/j.drudis.2014.08.017. PMID 25224017. Archived from the original on 28 August 2021. Retrieved 30 January 2022.
- 1 2 3 4 5 6 7 8 9 Kohrs R, Durieux ME (November 1998). "Ketamine: teaching an old drug new tricks". Anesthesia and Analgesia. 87 (5): 1186–93. doi:10.1213/00000539-199811000-00039. PMID 9806706.
- 1 2 Narita M, Yoshizawa K, Aoki K, Takagi M, Miyatake M, Suzuki T (September 2001). "A putative sigma1 receptor antagonist NE-100 attenuates the discriminative stimulus effects of ketamine in rats". Addiction Biology. 6 (4): 373–376. doi:10.1080/13556210020077091. PMID 11900615.
- ↑ Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR (April 2012). "Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo". European Neuropsychopharmacology. 22 (4): 308–17. doi:10.1016/j.euroneuro.2011.08.002. PMID 21911285.
- ↑ Appadu BL, Lambert DG (February 1996). "Interaction of i.v. anaesthetic agents with 5-HT3 receptors". British Journal of Anaesthesia. 76 (2): 271–3. doi:10.1093/bja/76.2.271. PMID 8777109.
- ↑ Peters JA, Malone HM, Lambert JJ (July 1991). "Ketamine potentiates 5-HT3 receptor-mediated currents in rabbit nodose ganglion neurones". British Journal of Pharmacology. 103 (3): 1623–5. doi:10.1111/j.1476-5381.1991.tb09837.x. PMC 1907797. PMID 1718520.
- ↑ Pharmaceutical Society of Australia; The Royal Australian College of General Practitioners; Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (2011). "2.1.1 IV General Anaesthetics". Australian Medicines Handbook 2011 (12th ed.). Adelaide: Australian Medicines Handbook Pty Ltd. p. 13. ISBN 978-0-9805790-4-8.
{{cite book}}: Cite has empty unknown parameter:|sectionurl=(help) - ↑ Ku SM, Han MH (July 2017). "HCN Channel Targets for Novel Antidepressant Treatment". Neurotherapeutics. 14 (3): 698–715. doi:10.1007/s13311-017-0538-7. PMC 5509632. PMID 28560710.
- ↑ Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. (May 2016). "NMDAR inhibition-independent antidepressant actions of ketamine metabolites". Nature. 533 (7604): 481–6. Bibcode:2016Natur.533..481Z. doi:10.1038/nature17998. PMC 4922311. PMID 27144355.
- ↑ Lodge D, Mercier MS (September 2015). "Ketamine and phencyclidine: the good, the bad and the unexpected". British Journal of Pharmacology. 172 (17): 4254–76. doi:10.1111/bph.13222. PMC 4556466. PMID 26075331.
- 1 2 3 Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ (June 2017). "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience. 8 (6): 1122–1134. doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- 1 2 Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, et al. (March 2017). "Administration of ketamine for unipolar and bipolar depression". International Journal of Psychiatry in Clinical Practice. 21 (1): 2–12. doi:10.1080/13651501.2016.1254802. PMID 28097909.
- 1 2 Bartova L, Vogl SE, Stamenkovic M, Praschak-Rieder N, Naderi-Heiden A, Kasper S, Willeit M (November 2015). "Combination of intravenous S-ketamine and oral tranylcypromine in treatment-resistant depression: A report of two cases". European Neuropsychopharmacology. 25 (11): 2183–4. doi:10.1016/j.euroneuro.2015.07.021. PMID 26302763.
- 1 2 Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. (January 2013). "Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors". European Journal of Pharmacology. 698 (1–3): 228–34. doi:10.1016/j.ejphar.2012.11.023. PMC 3534778. PMID 23183107.
- ↑ Jordan S, Chen R, Fernalld R, Johnson J, Regardie K, Kambayashi J, et al. (July 2006). "In vitro biochemical evidence that the psychotomimetics phencyclidine, ketamine and dizocilpine (MK-801) are inactive at cloned human and rat dopamine D2 receptors". European Journal of Pharmacology. 540 (1–3): 53–6. doi:10.1016/j.ejphar.2006.04.026. PMID 16730695.
- ↑ Seeman P (July 2004). "Comment on "Diverse psychotomimetics act through a common signaling pathway"". Science. 305 (5681): 180, author reply 180. doi:10.1126/science.1096072. PMID 15247457.
- ↑ The Role of Brain Dopamine. Springer Science & Business Media. 6 December 2012. pp. 23–. ISBN 978-3-642-73897-5. Archived from the original on 8 September 2017. Retrieved 9 August 2020.
- ↑ Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. (March 1994). "Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses". Archives of General Psychiatry. 51 (3): 199–214. doi:10.1001/archpsyc.1994.03950030035004. PMID 8122957.
- ↑ Hergovich N, Singer E, Agneter E, Eichler HG, Graselli U, Simhandl C, Jilma B (May 2001). "Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men. a randomized, double-blind, placebo-controlled trial". Neuropsychopharmacology. 24 (5): 590–3. doi:10.1016/S0893-133X(00)00194-9. PMID 11282259.
- 1 2 Rabiner EA (May 2007). "Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything there to be seen?". Journal of Psychopharmacology. 21 (3): 253–8. doi:10.1177/0269881107077767. PMID 17591653.
- ↑ Browne CA, Lucki I (December 2013). "Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants". Frontiers in Pharmacology. 4: 161. doi:10.3389/fphar.2013.00161. PMC 3873522. PMID 24409146.
- 1 2 3 4 Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T (August 2009). "Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug". Journal of Clinical Pharmacology. 49 (8): 957–64. doi:10.1177/0091270009337941. PMID 19546251. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- 1 2 Xu J, Lei H (December 2014). "Ketamine-an update on its clinical uses and abuses". CNS Neuroscience & Therapeutics. 20 (12): 1015–20. doi:10.1111/cns.12363. PMC 6493134. PMID 25417928.
- 1 2 3 4 5 Zanos P, Gould TD (April 2018). "Mechanisms of ketamine action as an antidepressant". Molecular Psychiatry. 23 (4): 801–811. doi:10.1038/mp.2017.255. PMC 5999402. PMID 29532791.
- 1 2 Zanos P, Thompson SM, Duman RS, Zarate CA, Gould TD (March 2018). "Convergent Mechanisms Underlying Rapid Antidepressant Action". CNS Drugs. 32 (3): 197–227. doi:10.1007/s40263-018-0492-x. PMC 6005380. PMID 29516301.
- ↑ Björkholm C, Monteggia LM (March 2016). "BDNF - a key transducer of antidepressant effects". Neuropharmacology. 102: 72–9. doi:10.1016/j.neuropharm.2015.10.034. PMC 4763983. PMID 26519901.
- ↑ Castrén E, Kojima M (January 2017). "Brain-derived neurotrophic factor in mood disorders and antidepressant treatments". Neurobiology of Disease. 97 (Pt B): 119–126. doi:10.1016/j.nbd.2016.07.010. hdl:10138/311483. PMID 27425886.
- ↑ Kim D, Cheong E, Shin HS (June 2018). "Overcoming Depression by Inhibition of Neural Burst Firing". Neuron. 98 (5): 878–879. doi:10.1016/j.neuron.2018.05.032. PMID 29879390.
- ↑ Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H (February 2018). "Ketamine blocks bursting in the lateral habenula to rapidly relieve depression". Nature. 554 (7692): 317–322. Bibcode:2018Natur.554..317Y. doi:10.1038/nature25509. PMID 29446381.
- 1 2 Garay R, Zarate CA, Cavero I, Kim YK, Charpeaud T, Skolnick P (October 2018). "The development of glutamate-based antidepressants is taking longer than expected". Drug Discovery Today. 23 (10): 1689–1692. doi:10.1016/j.drudis.2018.02.006. PMC 6211562. PMID 29501913.
- ↑ "Arketamine - Jiangsu Hengrui Medicine - AdisInsight". Archived from the original on 13 April 2021. Retrieved 9 August 2020.
- 1 2 Sleigh J, Harvey M, Voss L, Denny B (June 2014). "Ketamine – More mechanisms of action than just NMDA blockade". Trends in Anaesthesia and Critical Care. 4 (2–3): 76–81. doi:10.1016/j.tacc.2014.03.002.
- ↑ Lankenau SE, Sanders B, Bloom JJ, Hathazi D, Alarcon E, Tortu S, Clatts MC (March 2007). "First injection of ketamine among young injection drug users (IDUs) in three U.S. cities". Drug and Alcohol Dependence. 87 (2–3): 183–93. doi:10.1016/j.drugalcdep.2006.08.015. PMC 1852477. PMID 16979848.
- ↑ "Ketamine in Palliative Care" (PDF). Palliative Care Guidelines. Edinburgh: NHS Lothian, NHS Scotland, Health and Social Care Directorates, Scotland. August 2013 [August 2010]. Archived from the original (PDF) on 29 October 2013.
- 1 2 Haas DA, Harper DG (1992). "Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia". Anesthesia Progress. 39 (3): 61–8. PMC 2148758. PMID 1308374.
- 1 2 3 Cromhout A (April 2003). "Ketamine: its use in the emergency department". Emergency Medicine. 15 (2): 155–9. doi:10.1046/j.1442-2026.2003.00433.x. PMID 12675625. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- 1 2 3 Li JH, Vicknasingam B, Cheung YW, Zhou W, Nurhidayat AW, Jarlais DC, Schottenfeld R (2011). "To use or not to use: an update on licit and illicit ketamine use". Substance Abuse and Rehabilitation. 2 (1): 11–20. doi:10.2147/SAR.S15458. PMC 3846302. PMID 24474851.
- ↑ Krüger AD (1998). "[Current aspects of using ketamine in childhood]". Anaesthesiologie Und Reanimation (in Deutsch). 23 (3): 64–71. PMID 9707751.
- ↑ Chankvetadze B, Burjanadze N, Breitkreutz J, Bergander K, Bergenthal D, Kataeva O, Fröhlich R, Luftmann H, Blaschke G (2002). "Mechanistic study on the opposite migration order of the enantiomers of ketamine with α- and β-cyclodextrin in capillary electrophoresis". Journal of Separation Science. 25 (15–17): 1155–1166. doi:10.1002/1615-9314(20021101)25:15/17<1155::AID-JSSC1155>3.0.CO;2-M.
- ↑ Hakey P, Ouellette W, Zubieta J, Korter T (July 2008). "(S)-(+)-Ketamine hydro-chloride". Acta Crystallographica. Section E, Structure Reports Online. 64 (Pt 8): o1487. doi:10.1107/S1600536808021053. PMC 2962251. PMID 21203199.
- ↑ Ratti-Moberg E, Groth P, Aasen J, Arne J (1991). "The Absolute Configuration of Ketamine – A General Anaesthetic. The Crystal Structure of the (R,R)-Tartrate Salt of (−)-(S)-Ketamine". Acta Chemica Scandinavica. 45: 108–110. doi:10.3891/acta.chem.scand.45-0108.
- ↑ Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Feng N, Vollenweider FX, Minder EI, Rentsch K, Grampp T, Vonderschmitt DJ. Development of a gas chromatography-mass spectrometry method for determination of ketamine in plasma and its application to human samples. Ther. Drug Monit. 17: 95–100, 1995.
- ↑ Parkin MC, Turfus SC, Smith NW, Halket JM, Braithwaite RA, Elliott SP, Osselton MD, Cowan DA, Kicman AT. Detection of ketamine and its metabolites in urine by ultra high pressure liquid chromatography-tandem mass spectrometry. J. Chrom. B 876: 137–142, 2008.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 806–808.
- ↑ Clark, Michael R. (2011). Chronic Pain and Addiction. Basel, Switzerland: Karger AG. pp. 166–. ISBN 978-3-8055-9725-8.
- 1 2 3 Morris H, Wallach J (July 2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061. Archived from the original on 28 August 2021. Retrieved 9 August 2020.
- ↑ Corssen G, Domino EF (January–February 1966). "Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581". Anesthesia and Analgesia. 45 (1): 29–40. doi:10.1213/00000539-196601000-00007. PMID 5325977.
- 1 2 3 "Ketamine". Center for Substance Abuse Research (CESAR); University of Maryland, College Park. 29 October 2013. Archived from the original on 12 November 2013. Retrieved 27 July 2014.
- ↑ "'Ketamine – Just Say Neigh' Shirts Gets The Message Out". Trendhunter. 15 April 2009. Archived from the original on 19 August 2017. Retrieved 19 August 2017.
- ↑ History of non-medical use in literature references:
- ↑ Increased non-medical use references:
- Awuonda M (13 July 1996). "Swedes alarmed at ketamine misuse". The Lancet. 348 (9020): 122. doi:10.1016/S0140-6736(05)64628-4.
- Curran HV, Morgan C (April 2000). "Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later". Addiction. 95 (4): 575–90. doi:10.1046/j.1360-0443.2000.9545759.x. PMID 10829333.
- Gahlinger PM (June 2004). "Club drugs: MDMA, gamma-hydroxybutyrate (GHB), Rohypnol, and ketamine". American Family Physician. 69 (11): 2619–26. PMID 15202696. Archived from the original on 17 November 2015.
- Jansen KL (March 1993). "Non-medical use of ketamine". Bmj. 306 (6878): 601–2. doi:10.1136/bmj.306.6878.601. PMC 1676978. PMID 8461808.
- Joe-Laider & Hunt 2008
- 1 2 3 4 5 6 Joe-Laidler K, Hunt G (June 2008). "Sit Down to Float: The Cultural Meaning of Ketamine Use in Hong Kong". Addiction Research & Theory. 16 (3): 259–271. doi:10.1080/16066350801983673. PMC 2744071. PMID 19759834.
- ↑ Ketamine sold as "ecstasy" references:
- Tanner-Smith EE (July 2006). "Pharmacological content of tablets sold as "ecstasy": results from an online testing service" (PDF). Drug and Alcohol Dependence. 83 (3): 247–54. doi:10.1016/j.drugalcdep.2005.11.016. PMID 16364567. Archived (PDF) from the original on 4 March 2016.
- Copeland J, Dillon P (2005). "The health and psycho-social consequences of ketamine use". International Journal of Drug Policy. 16 (2): 122–31. doi:10.1016/j.drugpo.2004.12.003.
- Measham, Fiona; Parker, Howard; Aldridge, Judith (2001). Dancing on Drugs: Risk, Health and Hedonism in the British Club Scene. London: Free Association Books. ISBN 978-1-85343-512-6.
- ↑ Moore K, Measham F (2006). "Ketamine use: Minimising problems and maximising pleasure". Drugs and Alcohol Today. 6 (3): 29–32. doi:10.1108/17459265200600047.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 258–. ISBN 978-1-4757-2085-3. Archived from the original on 15 February 2017.
- 1 2 3 4 5 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 584–585. ISBN 978-3-88763-075-1.
- 1 2 "Esketamine". Archived from the original on 29 August 2017. Retrieved 28 August 2017.
- 1 2 Winter, Caroline (19 August 2015). "The Club Drug Cure: Ketamine's New Shot as a Depression Treatment". Bloomberg BusinessWeek. Archived from the original on 30 March 2017.
- ↑ Lawrence, Janna (19 March 2015). "The secret life of ketamine". Pharmaceutical Journal. Archived from the original on 15 August 2020. Retrieved 9 August 2020.
- ↑ Agres, Ted (3 December 2015). "Anesthesiologists Take Lead As Ketamine Clinics Proliferate". Anesthesiology News. Archived from the original on 9 January 2020. Retrieved 9 August 2020.
- ↑ Nulley, Dan (28 July 2015). "Australia Has Closed Its Most Controversial Ketamine Clinics". Vice. Archived from the original on 25 March 2017.
- ↑ Poisons Standard October 2015 "Archived copy". Archived from the original on 19 January 2016. Retrieved 6 January 2016.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Poisons Act 1964 http://www.slp.wa.gov.au/pco/prod/FileStore.nsf/Documents/MRDocument:26063P/$FILE/Poisons%20Act%201964%20-%20%5B09-f0-04%5D.pdf?OpenElement Archived 22 December 2015 at the Wayback Machine
- ↑ Legal status of ketamine in Canada references:
- "Statutes of Canada (S.C.) Controlled Drugs and Substances Act (S.C. 1996 c.19) Schedule I § 14". Justice Laws Website. Government of Canada. 12 June 2014. Archived from the original on 22 November 2013.
- "Order Amending Schedule I to the Controlled Drugs and Substances Act" (PDF). Canada Gazette Part II. Vol. 139, no. 19. 21 September 2005. p. 2130. Archived from the original (PDF) on 8 August 2014. Retrieved 2 August 2014.
- "Status of ketamine under CDSA". Canadian Society of Customs Brokers. 2 May 2005. Archived from the original on 10 August 2014. Retrieved 2 August 2014.
- ↑ "Government to tighten control on Ketamine", info.gov.hk (press release), Government of the Hong Kong Special Administrative, 22 November 2000, archived from the original on 26 June 2013, retrieved 2 August 2014
- ↑ Ho Wai-kin, Victor (2011). Criminal Law in Hong Kong. Kluwer Law International. p. 33. ISBN 9789041133069.
- ↑ Chang, Rich (4 December 2012). "Ketamine to be made a class-two drug: Official". Taipei Times. p. 3. Archived from the original on 8 August 2014.
- ↑ "Ketamine drug brought under 'Schedule X' to curb abuse". The Times of India. 7 January 2014. Archived from the original on 14 April 2014. Retrieved 2 August 2014.
- ↑ Deb Roy, Sumitra (30 December 2013). "Govt makes notorious 'date rape' drug ketamine harder to buy or sell". The Times of India. Archived from the original on 30 December 2013.
- ↑ "Club 'horse' drug to be outlawed". BBC News. 28 December 2005. Archived from the original on 3 September 2009. Retrieved 7 May 2010.
- ↑ Advisory Council on the Misuse of Drugs (ACMD); Baker, Norman (10 December 2013), Ketamine: A review of use and harm (PDF) (Policy paper), Crown copyright; Open Government Licence, archived (PDF) from the original on 28 February 2014, retrieved 22 January 2014.
- 1 2 Baker, Norman; (Minister for Crime Prevention); Home Office; United Kingdom (12 February 2014), Response to ACMD recommendation on ketamine (PDF) (Correspondence to Les Iverson [chair of]; Advisory Council on the Misuse of Drugs), Crown copyright; Open Government Licence, archived (PDF) from the original on 28 February 2014, retrieved 21 February 2014.
- ↑ Dixon, Hayley (12 February 2014). "Party drug ketamine to be upgraded to Class B". The Daily Telegraph. Archived from the original on 9 June 2014. Retrieved 2 August 2014.
- ↑ Marshall, DR; (Deputy Administrator); Drug Enforcement Administration; Department of Justice (13 July 1999). "Schedules of Controlled Substances: Placement of Ketamine into Schedule III [21 CFR Part 1308. Final Rule 99-17803]" (PDF). Rules and Regulations. Federal Register. 64 (133): 37673–5. Archived (PDF) from the original on 5 May 2015.
- ↑ Shelton, Gilbert (2008). The Freak Brothers Omnibus. London: Knockabout Comics. p. 144. ISBN 978-0-86166-159-6.
- ↑ Alltounian, Howard Sunny; Moore, Marcia (1978). Journeys into the Bright World. Rockport, Mass.: Para Research. ISBN 978-0-914918-12-7.
- ↑ Awuonda, Moussa (1 July 1996). "Swedes alarmed at ketamine misuse". The Lancet. 348 (9020): 122. doi:10.1016/S0140-6736(05)64628-4.
- ↑ Curran HV, Morgan C (April 2000). "Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later". Addiction. 95 (4): 575–90. doi:10.1046/j.1360-0443.2000.9545759.x. PMID 10829333.
- ↑ Gahlinger PM (June 2004). "Club drugs: MDMA, gamma-hydroxybutyrate (GHB), Rohypnol, and ketamine". American Family Physician. 69 (11): 2619–26. PMID 15202696.
- ↑ Jansen KL (March 1993). "Non-medical use of ketamine". Bmj (Clinical research ed.). 306 (6878): 601–2. doi:10.1136/bmj.306.6878.601. PMC 1676978. PMID 8461808.
- ↑ Measham, Fiona; Aldridge, Judith; Parker, Howard (2001). Dancing on drugs: risk, health and hedonism in the British club scene. London: Free Association. ISBN 978-1-85343-512-6.
- ↑ Saunders N, Heron L (1993). E for Ecstasy. London: N. Saunders. ISBN 978-0-9501628-8-1.
- ↑ See: Archived 17 April 2020 at the Wayback Machine for details online.
- ↑ Moore, Karenza; Measham, Fiona (1 January 2006). "Ketamine use: minimising problems and maximising pleasure". Drugs and Alcohol Today. 6 (3): 29–32. doi:10.1108/17459265200600047.
- 1 2 See Max Daly, 2014, "The Sad Demise of Nancy Lee, One of Britain's Ketamine Casualties," at Vice (online), 23 July 2014, see "The Sad Demise of Nancy Lee, One of Britain's Ketamine Casualties". 23 July 2014. Archived from the original on 7 June 2015. Retrieved 7 June 2015., accessed 7 June 2015.
- ↑ The Crown, 2013, "Drug related deaths involving ketamine in England and Wales," a report of the Mortality team, Life Events and Population Sources Division, Office for National Statistics, the Crown (U.K.), see "Archived copy". Archived from the original on 7 June 2015. Retrieved 7 June 2015.
{{cite web}}: CS1 maint: archived copy as title (link) and "Deaths Related to Drug Poisoning in England and Wales – Office for National Statistics". Archived from the original on 19 June 2015. Retrieved 7 June 2015., accessed 7 June 2015. - ↑ Dixon, Hayley (12 February 2014). "Ketamine death of public schoolgirl an 'act of stupidity which destroyed family'". Telegraph.co.uk. Archived from the original on 24 August 2017.
- 1 2 "Do you know... Ketamine". Knowledge Exchange. Toronto: Centre for Addiction and Mental Health. 2003. Archived from the original on 7 April 2014. Retrieved 27 July 2014.
- ↑ Giannini AJ, Loiselle RH, Giannini MC, Price WA (1985). "Phencyclidine and the dissociatives". Psychiatric Medicine. 3 (3): 197–217. PMID 2893430.
- ↑ Giannini AJ, Underwood NA, Condon M (November 2000). "Acute ketamine intoxication treated by haloperidol: a preliminary study". American Journal of Therapeutics. 7 (6): 389–91. doi:10.1097/00045391-200007060-00008. PMID 11304647.
- ↑ Giannini AJ (1999). Drug Abuse. Los Angeles: Health Information Press. p. 104. ISBN 978-1-885987-11-2.
- ↑ References for recreational use in literature:
- Lilly, John Cunningham (1997). The Scientist: A Metaphysical Autobiography. Berkeley, CA: Ronin. pp. 144–. ISBN 978-0-914171-72-0.
- Kelly, Kit (2001). The Little Book of Ketamine. Ronin. pp. 23, 40–45, 46–51, ibid. ISBN 978-1-57951-121-0.
{{cite book}}: CS1 maint: ref duplicates default (link) - Alltounian, Howard Sunny; Moore, Marcia (1978). Journeys Into the Bright World. Rockport, MA: Para Research. ISBN 978-0-914918-12-7.
- Palmer, Cynthia; Horowitz, Michael (2000). Sisters of the Extreme: Women Writing on the Drug Experience. Inner Traditions. pp. 254–258, ibid. ISBN 978-0-89281-757-3.
- Turner DM (1994). The Essential Psychedelic Guide. San Francisco: Panther Press. ISBN 978-0-9642636-1-1.
- ↑ Jansen, Karl (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. pp. 50, 89. ISBN 978-0-9660019-3-8.
- ↑ Woodard, D., “The Ketamine Necromance” Archived 24 June 2021 at the Wayback Machine, in A. Parfrey, Apocalypse Culture II (Los Angeles: Feral House, 2000), pp. 288–295.
- ↑ Jansen, Karl (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. p. 24. ISBN 978-0-9660019-3-8.
- 1 2 3 4 5 6 7 8 9 10 "Center for Substance Abuse Research: Ketamine Terminology". Archived from the original on 12 November 2013. Retrieved 3 January 2012.
- 1 2 Jansen, Karl (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. p. 26. ISBN 978-0-9660019-3-8.
- 1 2 Jansen, Karl (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. p. 55. ISBN 978-0-9660019-3-8.
- 1 2 "Research Report on Hallucinogens and Dissociatives" (PDF). NIDA. Archived (PDF) from the original on 28 January 2012. Retrieved 11 January 2012.
- 1 2 "DEA Office of Diversion Control: Ketamine". Archived from the original on 15 December 2011. Retrieved 3 January 2012.
- 1 2 3 "Ketamine: A fact sheet (National Clearinghouse for Alcohol and Drug Information)" (PDF). Archived from the original (PDF) on 26 April 2012. Retrieved 3 January 2012.
- 1 2 Tellier PP (September 2002). "Club drugs: is it all ecstasy?". Pediatric Annals. 31 (9): 550–6. doi:10.3928/0090-4481-20020901-07. PMID 12271739.
- ↑ Nutt, David (2012). Drugs – Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge. ISBN 978-1-906860-16-5.
- ↑ Chakraborty K, Neogi R, Basu D (June 2011). "Club drugs: review of the 'rave' with a note of concern for the Indian scenario". The Indian Journal of Medical Research. 133 (6): 594–604. PMC 3135986. PMID 21727657.
- ↑ "Trends in Use of Various Drugs, Table 2" (PDF). Monitoring the Future. Archived (PDF) from the original on 13 January 2012. Retrieved 17 January 2012.
- 1 2 "Use of Specific Hallucinogens: 2006". The NSDUH Report. Substance Abuse and Mental Health Services Administration. Archived from the original on 13 February 2012. Retrieved 17 January 2012.
- ↑ "Drug Abuse Warning Network, 2009: National Estimates of Drug-Related Emergency Department Visits" (PDF). HHS Publication No. (SMA) 11-4659, DAWN Series D-35. Substance Abuse and Mental Health Services Administration. Archived from the original (PDF) on 12 March 2012. Retrieved 17 January 2012.
- 1 2 "SI.com – The Mexican Connection – Jul 18, 2007". CNN. Archived from the original on 4 June 2011. Retrieved 7 May 2010.
- ↑ "Untitled-59" (PDF). Archived (PDF) from the original on 14 May 2015. Retrieved 9 August 2020.
- ↑ Kalsi SS, Wood DM, Dargan PI (April 2011). "The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use". Emerging Health Threats Journal. 4: 7107. doi:10.3402/ehtj.v4i0.7107. PMC 3168228. PMID 24149025.
- ↑ Błachut M, Sołowiów K, Janus A, Ruman J, Cekus A, Matysiakiewicz J, Hese RT (September–October 2009). "[A case of ketamine dependence]". Psychiatria Polska. 43 (5): 593–9. PMID 20214100.
- ↑ Greenwald, Glenn. "Drug Decriminalization in Portugal" (PDF). CATO Institute. Archived (PDF) from the original on 5 January 2012. Retrieved 12 January 2012.
- ↑ "2010 National Drug Strategy Household Survey report". Australian Institute of Health and Welfare. Archived from the original on 8 February 2014. Retrieved 11 January 2012.
- ↑ "The Ketamine Connection". Archived from the original on 14 April 2021. Retrieved 9 August 2020.
- ↑ Cheung, Yuet. "Drug Policy and Harm Reduction in Hong Kong: A Socio-Historical Examination*" (PDF). Archived (PDF) from the original on 18 October 2011. Retrieved 12 January 2012.
- ↑ "CRDA Drug Statistics". Archived from the original on 1 July 2010. Retrieved 12 January 2012.
- 1 2 "CRDA Fifty-ninth Report (2000–2009)" (PDF). Archived (PDF) from the original on 14 April 2014. Retrieved 12 January 2012.
- 1 2 Chen WJ, Fu TC, Ting TT, Huang WL, Tang GM, Hsiao CK, Chen CY (January 2009). "Use of ecstasy and other psychoactive substances among school-attending adolescents in Taiwan: national surveys 2004-2006". BMC Public Health. 9 (1): 27. doi:10.1186/1471-2458-9-27. PMC 2636802. PMID 19159468.
- ↑ Wang SH, Chen WC, Lew-Ting CY, Chen CY, Chen WJ (January 2010). "Running away experience and psychoactive substance use among adolescents in Taiwan: multi-city street outreach survey". BMC Public Health. 10 (1): 29. doi:10.1186/1471-2458-10-29. PMC 2823700. PMID 20089181.
- ↑ Wang YC, Lee CM, Lew-Ting CY, Hsiao CK, Chen DR, Chen WJ (October 2005). "Survey of substance use among high school students in Taipei: web-based questionnaire versus paper-and-pencil questionnaire". The Journal of Adolescent Health. 37 (4): 289–95. doi:10.1016/j.jadohealth.2005.03.017. PMID 16182139.
- ↑ Lua AC, Lin HR, Tseng YT, Hu AR, Yeh PC (September 2003). "Profiles of urine samples from participants at rave party in Taiwan: prevalence of ketamine and MDMA abuse". Forensic Science International. 136 (1–3): 47–51. doi:10.1016/S0379-0738(03)00261-5. PMID 12969619.
- ↑ Krupitsky EM, Grineko AY, Berkaliev TN, Paley AI, Tetrov UN, Mushkov KA, Borodikin YS (1992). "The combination of psychedelic and aversive approaches in alcoholism treatment". Alcoholism Treatment Quarterly. 9 (1): 99–105. doi:10.1300/J020V09N01_09.
- ↑ Krupitsky EM, Grinenko AY (1997). "Ketamine psychedelic therapy (KPT): a review of the results of ten years of research". Journal of Psychoactive Drugs. 29 (2): 165–83. doi:10.1080/02791072.1997.10400185. PMID 9250944.
- ↑ Krupitsky, Evgeny; Kolp, Eli (2007). "Ch. 6: Ketamine Psychedelic Psychotherapy". In Winkelman, Michael J.; Roberts, Thomas B. (eds.). Psychedelic Medicine: New Evidence for Hallucinogens as Treatments. Vol. 2. Westport, CT: Praeger. ISBN 978-0-275-99023-7.
- ↑ Robertson SA, Taylor PM (October 2004). "Pain management in cats--past, present and future. Part 2. Treatment of pain--clinical pharmacology". Journal of Feline Medicine and Surgery. 6 (5): 321–33. doi:10.1016/j.jfms.2003.10.002. PMID 15363764.
- ↑ Lamont LA (November 2008). "Adjunctive analgesic therapy in veterinary medicine". The Veterinary Clinics of North America. Small Animal Practice. 38 (6): 1187–203, v. doi:10.1016/j.cvsm.2008.06.002. PMID 18954680.
- ↑ Stunkard JA, Miller JC (September 1974). "An outline guide to general anesthesia in exotic species". Veterinary Medicine, Small Animal Clinician. 69 (9): 1181–6. PMID 4604091.
- ↑ Riviere JE, Papich MG (2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 200. ISBN 978-1-118-68590-7. Archived from the original on 24 August 2017.
- ↑ Standard Operating Procedure No. 1 Anesthesia and Analgesia in Rodents, Washington College, 2012, pp. 1–2, archived from the original on 4 August 2013, retrieved 27 November 2015
- ↑ Woodall AJ, McCrohan CR (December 2000). "Excitatory actions of propofol and ketamine in the snail Lymnaea stagnalis". Comparative Biochemistry and Physiology. Toxicology & Pharmacology. 127 (3): 297–305. doi:10.1016/S0742-8413(00)00155-9. PMID 11246501.
External links
| External sites: |
|
|---|---|
| Identifiers: |
|