Fumarase deficiency
| Fumarase deficiency | |
|---|---|
| Other names: Fumarate hydratase deficiency [1] | |
.jpg.webp) | |
| Summary of the compensatory pathways used when simulating fumarase deficiency with the objective function of maximum ATP[2] | |
Fumarase deficiency (or fumaric aciduria) is an exceedingly rare autosomal recessive metabolic disorder in the Krebs cycle, characterized by a deficiency of the enzyme fumarate hydratase, which causes a buildup of fumaric acid in the urine and a deficiency of malate. Only 13 cases were known worldwide in 1990, after which a cluster of 20 cases was documented in a community in Arizona that has practiced successive endogamy.
Signs and symptoms
Fumarase deficiency causes encephalopathy,[3] severe intellectual disabilities, unusual facial features, brain malformation, and epileptic seizures[4] due to an abnormally low amount of fumarase in cells. It can initially present with polyhydramnios on prenatal ultrasound. Affected neonates may demonstrate nonspecific signs of poor feeding and hypotonia. Laboratory findings in neonates may indicate polycythemia, leukopenia, or neutropenia. As they age, neurological deficits begin to manifest with seizures, dystonias, and severe developmental delay.[5]
Cause
Fumarase deficiency is caused by a mutation in the fumarate hydratase (FH) gene in humans, which encodes the enzyme that converts fumarate to malate in the mitochondria. Other mutant alleles of the FH gene, located on human Chromosome 1 at position 1q42.1, cause multiple cutaneous and uterine leiomyomata, hereditary leiomyomatosis and renal cell cancer.[6]
Fumarase deficiency is one of the few known deficiencies of the Krebs cycle or tricarboxylic acid cycle, the main enzymatic pathway of cellular aerobic respiration.[7]
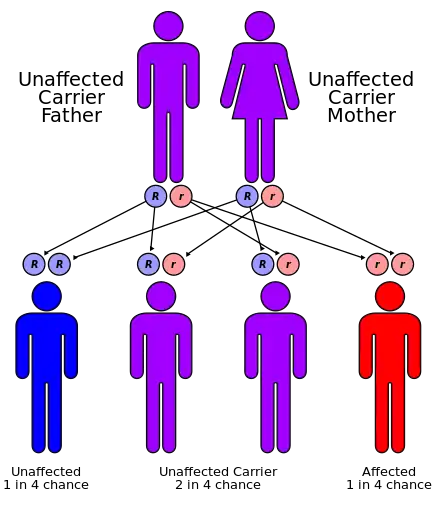
The condition is an autosomal recessive disorder,[8] and it is therefore usually necessary for an affected individual to receive the mutant allele from both parents. A number of children diagnosed with the disorder have been born to parents who were first cousins.[9][10]
It can also be associated with uniparental isodisomy.[11]
Diagnosis
In terms of the diagnosis of Fumarase deficiency, we find that confirmation is done via measuring fumarate hydratase activity in leukocytes. Additionally a brain MRI may be done in the evaluation process[12]
Treatment
The available management of Fumarase deficiency is symptomatic only, these include the following:[12]
- Physical therapy
- Special education
- Occupational therapy
Epidemiology
Fumarase deficiency is extremely rare – until around 1990 there had only been 13 diagnosed and identified cases worldwide.
A cluster of 20 cases has since been documented in the twin towns of Colorado City, Arizona and Hildale, Utah, both of which were formerly known as "Short Creek" (or the Short Creek Community). The two towns combine to form a community of 10,000 members of the Fundamentalist Church of Jesus Christ of Latter Day Saints (FLDS) who have a history of practicing successive endogamy, or marriage within their own communities.[13][14][15][16] Nicknamed "Polygamist's Down's", the syndrome has been blamed on cousin marriage, but in a larger sense is related to the reproductive isolation of a community among whom 85% are blood relatives of John Y. Barlow and/or Joseph Smith Jessop (the cofounders of the Short Creek Community).[14]
Since the initial cluster from FLDS communities were reported, it is now estimated that there are 100 documented cases worldwide.[17]
See also
- Hereditary leiomyomatosis and renal cell cancer
- Founder effect
References
- ↑ "Fumarase deficiency | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 8 April 2019. Retrieved 8 April 2019.
- ↑ Smith, Anthony C.; Eyassu, Filmon; Mazat, Jean-Pierre; Robinson, Alan J. (25 November 2017). "MitoCore: a curated constraint-based model for simulating human central metabolism". BMC systems biology. 11 (1): 114. doi:10.1186/s12918-017-0500-7. ISSN 1752-0509. Archived from the original on 19 December 2022. Retrieved 3 July 2023.
- ↑ Bayley, Jean-Pierre; Launonen, Virpi; Tomlinson, Ian P.M. (25 March 2008). "The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency". BMC Med. Genet. 9 (1): 20. doi:10.1186/1471-2350-9-20. PMC 2322961. PMID 18366737.
- ↑ Kerrigan, John F.; Aleck, Kirk A.; Tarby, Theodore J.; Bird, C. Roger; Heidenreich, Randall A. (May 2000) [3 May 2001]. "Fumaric aciduria: clinical and imaging features". Ann. Neurol. 47 (5): 583–8. doi:10.1002/1531-8249(200005)47:5<583::AID-ANA5>3.0.CO;2-Y. PMID 10805328. S2CID 10448322.
- ↑ Ewbank, Clifton; Kerrigan, John F.; Aleck, Kirk (April 4, 2013) [July 5, 2006]. "Fumarate Hydratase Deficiency". GeneReviews. Seattle WA: University of Washington. PMID 20301679. Archived from the original on January 28, 2023. Retrieved April 26, 2023.
- ↑ Online Mendelian Inheritance in Man (OMIM): Fumarase Deficiency - 606812
- ↑ Devlin, Thomas M. (2006). Textbook of biochemistry: with clinical correlations. New York: John Wiley. p. 546. ISBN 978-0-471-67808-3.
- ↑ Gellera, C.; Uziel, G.; Rimoldi, M.; Zeviani, M.; Laverda, A.; Carrara, F.; DiDonato, S. (March 1990). "Fumarase deficiency is an autosomal recessive encephalopathy affecting both the mitochondrial and the cytosolic enzymes". Neurology. 40 (3 Part 1): 495–499. doi:10.1212/wnl.40.3_part_1.495. PMID 2314594. S2CID 1292556.
- ↑ Petrova-Benedict, R.; Robinson, B.H.; Stacey, T.E.; Mistry, J.; Chalmers, R.A. (1987). "Deficient fumarase activity in an infant with fumaricacidemia and its distribution between the different forms of the enzyme seen on isoelectric focusing". Am. J. Hum. Genet. 40 (3): 257–266. PMC 1684096. PMID 3578275.
- ↑ Bourgeron, T.; Chretien, D.; Poggi-Bach, J.; Doonan, S.; Rabier, D.; Letouzé, P.; Munnich, A.; Rötig, A.; Landrieu, P.; Rustin, P. (June 1994). "Mutation of the fumarase gene in two siblings with progressive encephalopathy and fumarase deficiency". J. Clin. Invest. 93 (6): 2514–2518. doi:10.1172/JCI117261. PMC 294471. PMID 8200987.
- ↑ Zeng, Wen-Qi; Gao, Hanlin; Brueton, Louise; Hutchin, Tim; Gray, George; Chakrapani, Anupam; Olpin, Simon; Shih, Vivian E. (1 May 2006) [30 March 2006]. "Fumarase deficiency caused by homozygous P131R mutation and paternal partial isodisomy of chromosome 1". Am. J. Med. Genet. A. 140A (9): 1004–1009. doi:10.1002/ajmg.a.31186. PMID 16575891. S2CID 38553151.
- 1 2 RESERVED, INSERM US14-- ALL RIGHTS. "Orphanet: Fumaric aciduria". www.orpha.net. Archived from the original on 7 October 2022. Retrieved 3 July 2023.
- ↑ Dougherty, John (December 29, 2005). "Forbidden Fruit: Inbreeding among polygamists along the Arizona-Utah border is producing a caste of severely retarded and deformed children". The Phoenix New Times News. p. 2. Archived from the original on 2015-04-20. Retrieved 2008-04-16.
- 1 2 "Mormon Sect's Polygamy Causes Most Of The World's Fumarase Deficiency Cases". Digital Journal. 2007-06-14. Archived from the original on 2019-05-23. Retrieved 2023-04-26.
- ↑ Hollenhorst, John (February 8, 2006). "Birth defect is plaguing children in FLDS towns: Fumarase Deficiency afflicts 20, is linked to marriages of close Kin". Deseret News. Archived from the original on August 9, 2008. Retrieved April 26, 2023.
- ↑ Szep, Jason (June 14, 2007). "Polygamist community faces rare genetic disorder". Reuters. Archived from the original on September 12, 2012. Retrieved April 26, 2023.
- ↑ Fumarase deficiency Archived 2023-03-22 at the Wayback Machine MedlinePlus accessed via Internet June 20, 2022
Further reading
- Pithukpakorn, Manop; Toro, Jorge R. (November 2, 2010) [July 31, 2006]. "Hereditary Leiomyomatosis and Renal Cell Cancer". GeneReviews. Seattle WA: University of Washington. PMID 20301430. Archived from the original on July 9, 2023. Retrieved April 26, 2023.
External links
| Classification | |
|---|---|
| External resources |
|