Immunoglobulin therapy
| Names | |
|---|---|
| Trade names | Flebogamma, Gammagard, Hizentra, others |
| Other names | normal human immunoglobulin (HNIG), human normal immunoglobulin (HNIG) |
| Clinical data | |
| Main uses | Primary immunodeficiency, immune thrombocytopenic purpura, chronic inflammatory demyelinating polyneuropathy, Kawasaki disease, certain cases of HIV/AIDS and measles, Guillain-Barré syndrome[1] |
| Side effects | Pain at the site of injection, muscle pain, allergic reactions[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous (IV), intramuscular (IM), subcutaneous (SC) |
| Defined daily dose | not established[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
Immunoglobulin therapy, also known as normal human immunoglobulin (NHIG), is the use of a mixture of antibodies (immunoglobulins) to treat a number of health conditions.[1][3] These conditions include primary immunodeficiency, immune thrombocytopenic purpura, chronic inflammatory demyelinating polyneuropathy, Kawasaki disease, certain cases of HIV/AIDS and measles, Guillain-Barré syndrome, and certain other infections when a more specific immunoglobulin is not available.[1] Depending on the formulation it can be given by injection into muscle, a vein, or under the skin.[1] The effects last a few weeks.[3]
Common side effects include pain at the site of injection, muscle pain, and allergic reactions.[1] Other severe side effects include kidney problems, anaphylaxis, blood clots, and red blood cell breakdown.[1] Use is not recommended in people with some types of IgA deficiency.[1] Use appears to be relatively safe during pregnancy.[1] Human immunoglobulin is made from human blood plasma.[1] It contains antibodies against many viruses.[3]
Human immunoglobulin therapy first occurred in the 1930s and a formulation for injection into a vein was approved for medical use in the United States in 1981.[4] It is on the World Health Organization's List of Essential Medicines.[5] Each formulation of product is somewhat different.[3] In the United Kingdom a dose cost the NHS between 11 and 1,200 pounds depending on the type and amount.[3] A number of specific immunoglobulin formulations are also available including for hepatitis B, rabies, tetanus, varicella infection, and Rh positive blood exposure.[3]
Medical uses
Immunoglobulin therapy is used in a variety of conditions, many of which involve decreased or abolished antibody production capabilities, which range from a complete absence of multiple types of antibodies, to IgG subclass deficiencies (usually involving IgG2 or IgG3), to other disorders in which antibodies are within a normal quantitative range, but lacking in quality - unable to respond to antigens as they normally should – resulting in an increased rate or increased severity of infections. In these situations, immunoglobulin infusions confer passive resistance to infection on their recipients by increasing the quantity/quality of IgG they possess. Immunoglobulin therapy is also used for a number of other conditions, including in many autoimmune disorders such as dermatomyositis in an attempt to decrease the severity of symptoms. Immunoglobulin therapy is also used in some treatment protocols for secondary immunodeficiencies such as human immunodeficiency virus (HIV), some autoimmune disorders (such as immune thrombocytopenia and Kawasaki disease), some neurological diseases (multifocal motor neuropathy, stiff person syndrome, multiple sclerosis and myasthenia gravis) some acute infections and some complications of organ transplantation.[6]
Immunoglobulin therapy is especially useful in some acute infection cases such as pediatric HIV infection and is also considered the standard of treatment for some autoimmune disorders such as Guillain–Barré syndrome.[7][8] The high demand which coupled with the difficulty of producing immunoglobulin in large quantities has resulted in increasing global shortages, usage limitations and rationing of immunoglobulin.[9]
Different national bodies and medical associations have established varying standards for the use of immunoglobulin therapy.
United Kingdom
The United Kingdom's National Health Service recommends the routine use of immunoglobulin for a variety of conditions including primary immunodeficiencies and a number of other conditions, but recommends against the use of immunoglobulin in sepsis (unless a specific toxin has been identified), multiple sclerosis, neonatal sepsis, and pediatric HIV.[10]
United States
The American Academy of Allergy, Asthma, and Immunology most strongly supports the use of immunoglobulin for primary immunodeficiencies, while noting that such usage actually accounts for a minority of usage and acknowledging that immunoglobulin supplementation can be appropriately used for a number of other conditions,[11] including neonatal sepsis (citing a sixfold decrease in mortality), considered in cases of HIV (including pediatric HIV), considered as a second line treatment in relapsing-remitting multiple sclerosis, but recommending against its use in such conditions as chronic fatigue syndrome, PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection) until further evidence to support its use is found (though noting that it may be useful in PANDAS patients with an autoimmune component), cystic fibrosis, and a number of other conditions.[6]
Canada
The National Advisory Committee on Blood and Blood Products of Canada (NAC) and Canadian Blood Services have also developed their own separate set of guidelines for the appropriate use of immunoglobulin therapy, which strongly support the use of immunoglobulin therapy in primary immunodeficiencies and some complications of HIV, while remaining silent on the issues of sepsis, multiple sclerosis, and chronic fatigue syndrome.[12]
Australia
The Australian Red Cross Blood Service developed their own guidelines for the appropriate use of immunoglobulin therapy in 1997.[13] Immunoglobulin is funded under the National Blood Supply and indications are classified as either an established or emerging therapeutic role or conditions for which immunoglobulin use is in exceptional circumstances only.[14]
Subcutaneous immunoglobulin access programs have been developed to facilitate hospital based programs.[15] In Australia subcutaneous immunoglobulin is approved for primary immunodeficiency disease, specific antibody disease, acquired or secondary hypogammaglobulinemia and chronic inflammatory demyelinating polyneuropathy.
European Union
Brands include HyQvia (human normal immunoglobulin), Privigen (human normal immunoglobulin (IVIg)), Hizentra (human normal immunoglobulin (SCIg)), Kiovig (human normal immunoglobulin), and Flebogamma DIF (human normal immunoglobulin). [16][17][18][19]
In the EU human normal immunoglobulin (SCIg) (Hizentra) is used in people whose blood does not contain enough antibodies (proteins that help the body to fight infections and other diseases), also known as immunoglobulins.[17] It is used to treat the following conditions:
- primary immunodeficiency syndromes (PID, when people are born with an inability to produce enough antibodies);[17]
- low levels of antibodies in the blood in people with chronic lymphocytic leukaemia (a cancer of a type of white blood cell) or myeloma (a cancer of another type of white blood cell) and who have frequent infections;[17]
- low levels of antibodies in the blood in people before or after allogeneic haematopoietic stem cell transplantation (a procedure where the patient's bone marrow is cleared of cells and replaced by stem cells from a donor);[17]
- chronic inflammatory demyelinating polyneuropathy (CIDP). In this rare disease, the immune system (the body's defence system) works abnormally and destroys the protective covering over the nerves.[17]
It is indicated for replacement therapy in adults and children in primary immunodeficiency syndromes such as:
- congenital agammaglobulinaemia and hypogammaglobulinaemia (low levels of antibodies);[17]
- common variable immunodeficiency;[17]
- severe combined immunodeficiency;[17]
- immunoglobulin-G-subclass deficiencies with recurrent infections;[17]
- replacement therapy in myeloma or chronic lymphocytic leukaemia with severe secondary hypogammaglobulinaemia and recurrent infections.[17]
Flebogamma DIF is indicated for the replacement therapy in adults, children and adolescents (0–18 years) in:
- primary immunodeficiency syndromes with impaired antibody production;[20]
- hypogammaglobulinaemia (low levels of antibodies) and recurrent bacterial infections in patients with chronic lymphocytic leukaemia (a cancer of a type of white blood cell), in whom prophylactic antibiotics have failed;[20]
- hypogammaglobulinaemia (low levels of antibodies) and recurrent bacterial infections in plateau-phase-multiple-myeloma (another cancer of a type of white blood cell) patients who failed to respond to pneumococcal immunisation;[20]
- hypogammaglobulinaemia (low levels of antibodies) in patients after allogenic haematopoietic-stem-cell transplantation (HSCT) (when the patient receives stem cells from a matched donor to help restore the bone marrow);[20]
- congenital acquired immune deficiency syndrome (AIDS) with recurrent bacterial infections.[20]
and for the immunomodulation in adults, children and adolescents (0–18 years) in:
- primary immune thrombocytopenia (ITP), in patients at high risk of bleeding or prior to surgery to correct the platelet count;[20]
- Guillain Barré syndrome, which causes multiple inflammations of the nerves in the body;[20]
- Kawasaki disease, which causes multiple inflammation of several organs in the body.[20]
Dosage
The defined daily dose is not established (Human Immunoglobulin)[2]
Side effects
.png.webp)
Although immunoglobulin is frequently used for long periods of time and is generally considered safe, immunoglobulin therapy can have severe adverse effects, both localized and systemic. Subcutaneous administration of immunoglobulin is associated with a lower risk of both systemic and localized risk when compared to intravenous administration (hyaluronidase-assisted subcutaneous administration is associated with a greater frequency of adverse effects than traditional subcutaneous administration but still a lower frequency of adverse effects when compared to intravenous administration). Patients who are receiving immunoglobulin and experience adverse events are sometimes recommended to take acetaminophen and diphenhydramine before their infusions to reduce the rate of adverse effects. Additional premedication may be required in some instances (especially when first getting accustomed to a new dosage), prednisone or another oral steroid.
Local side effects of immunoglobulin infusions most frequently include an injection site reaction (reddening of the skin around the injection site), itching, rash, and hives.[21] Less serious systemic side effects to immunoglobulin infusions include an increased heart rate, hyper or hypotension, an increased body temperature, diarrhea, nausea, abdominal pain, vomiting, arthralgia or myalgia, dizziness, headache, fatigue, fever, and pain.[21]
Serious side effects of immunoglobulin infusions include chest discomfort or pain, myocardial infarction, tachycardia, hyponatremia, hemolysis, hemolytic anemia, thrombosis, hepatitis, anaphylaxis, backache, aseptic meningitis, acute kidney injury, hypokalemic nephropathy, pulmonary embolism, and transfusion related acute lung injury.[21] There is also a small chance that even given the precautions taken in preparing immunoglobulin preparations, an immunoglobulin infusion may pass a virus to its recipient.[21] Some immunoglobulin solutions also contain isohemagglutinins, which in rare circumstances can cause hemolysis by the isohemagglutinins triggering phagocytosis.[22]
In the case of less serious side effects, a patient's infusion rate can be adjusted downwards until the side effects become tolerable, while in the case of more serious side effects, emergency medical attention should be sought.[23]
Immunoglobulin therapy also interferes with the ability of the body to produce a normal immune response to an attenuated live virus vaccine for up to a year,[21] can result in falsely elevated blood glucose levels,[21] and can interfere with many of the IgG-based assays often used to diagnose a patient with a particular infection.[24]
Routes of administration
1950s – intramuscular
After immunoglobulin therapy's discovery and description in Pediatrics in 1952, weekly intramuscular injections of immunoglobulin (IMIg) were the norm until intravenous formulations (IVIg) began to be introduced in the 1980s.[25] During the mid and late 1950s, one-time IMIG injections were a common public health response to outbreaks of polio before the widespread availability of vaccines. Intramuscular injections were extremely poorly tolerated due to their extreme pain and poor efficacy – rarely could intramuscular injections alone raise plasma immunoglobulin levels enough to make a clinically meaningful difference.[25]
1980s – intravenous
Intravenous formulations began to be approved in the 1980s, which represented a significant improvement over intramuscular objections, as they allowed for a sufficient amount of immunoglobulin to be injected to reach clinical efficacy, although they still had a fairly high rate of adverse effects (though the addition of stabilizing agents reduced this further).[25]
1990s - subcutaneous
The first description of a subcutaneous route of administration for immunoglobulin therapy dates back to 1980,[26] but for many years subcutaneous administration was considered to be a secondary choice, only to be considered when peripheral venous access was no longer possible or tolerable.[25]
During the late 1980s and early 1990s, it became obvious that for at least a subset of patients the systemic adverse events associated with intravenous therapy were still not easily tolerable, and more doctors began to experiment with subcutaneous immunoglobulin administration, culminating in an ad hoc clinical trial in Sweden of 3000 subcutaneous injections administered to 25 adults (most of whom had previously experienced systemic adverse effects with IMIg or IVIg), where no infusion in the ad hoc trial resulted in a severe systemic adverse reaction, and most subcutaneous injections were able to be administered in non-hospital settings, allowing for considerably more freedom for the people involved.[25]
In the later 1990s, large-scale trials began in Europe to test the feasibility of subcutaneous immunoglobulin administration, although it was not until 2006 that the first subcutaneous-specific preparation of immunoglobulin was approved by a major regulatory agency (Vivaglobin, which was voluntarily discontinued in 2011).[25][27] A number of other trade names of subcutaneous immunoglobulin have since been approved, although some small-scale studies have indicated that a particular cohort of patients with Common variable immunodeficiency (CVID) may suffer intolerable side effects with subcutaneous immunoglobulin (SCIg) that they do not with intravenous immunoglobulin (IVIg).[25]
Although intravenous was the preferred route for immunoglobulin therapy for many years, in 2006, the US Food and Drug Administration (FDA) approved the first preparation of immunoglobulin that was designed exclusively for subcutaneous use.[25]
Mechanism of action
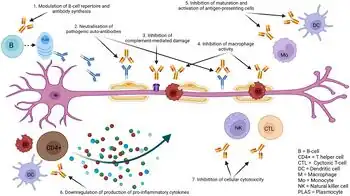
The precise mechanism by which immunoglobulin therapy suppresses harmful inflammation is likely multifactorial. For example, it has been reported that immunoglobulin therapy can block Fas-mediated cell death.[29]
Perhaps a more popular theory is that the immunosuppressive effects of immunoglobulin therapy are mediated through IgG's Fc glycosylation. By binding to receptors on antigen presenting cells, IVIG can increase the expression of the inhibitory Fc receptor, FcgRIIB, and shorten the half-life of auto-reactive antibodies.[30][31][32] The ability of immunoglobulin therapy to suppress pathogenic immune responses by this mechanism is dependent on the presence of a sialylated glycan at position CH2-84.4 of IgG.[30] Specifically, de-sialylated preparations of immunoglobulin lose their therapeutic activity and the anti-inflammatory effects of IVIG can be recapitulated by administration of recombinant sialylated IgG1 Fc.[30]
There are several other proposed mechanisms of action and the actual primary targets of immunoglobulin therapy in autoimmune disease are still being elucidated. Some believe that immunoglobulin therapy may work via a multi-step model where the injected immunoglobulin first forms a type of immune complex in the patient.[33] Once these immune complexes are formed, they can interact with Fc receptors on dendritic cells,[34] which then mediate anti-inflammatory effects helping to reduce the severity of the autoimmune disease or inflammatory state.
Other proposed mechanisms include the possibility that donor antibodies may bind directly with the abnormal host antibodies, stimulating their removal; the possibility that IgG stimulates the host's complement system, leading to enhanced removal of all antibodies, including the harmful ones; and the ability of immunoglobulin to block the antibody receptors on immune cells (macrophages), leading to decreased damage by these cells, or regulation of macrophage phagocytosis. Indeed, it is becoming more clear that immunoglobulin can bind to a number of membrane receptors on T cells, B cells, and monocytes that are pertinent to autoreactivity and induction of tolerance to self.[30][35]
A recent report stated that immunoglobulin application to activated T cells leads to their decreased ability to engage microglia. As a result of immunoglobulin treatment of T cells, the findings showed reduced levels of tumor necrosis factor-alpha and interleukin-10 in T cell-microglia co-culture. The results add to the understanding of how immunoglobulin may affect inflammation of the central nervous system in autoimmune inflammatory diseases.[36]
Society and culture
Brand names
As biologicals, various trade names of immunoglobulin products are not necessarily interchangeable, and care must be exercised when changing between them.[37] Trade names of intravenous immunoglobulin formulations include Flebogamma, Gamunex, Privigen, Octagam and Gammagard, while trade names of subcutaneous formulations include Cutaquig, Cuvitru, HyQvia, Hizentra,[17][38][39] Gamunex-C, and Gammaked.[40]
Supply issues
The United States is one of a handful of countries that allow plasma donors to be paid, meaning that the US supplies much of the plasma-derived medicinal products (including immunoglobulin) used across the world, including more than 50% of the European Union's supply.[41] The Council of Europe has officially endorsed the idea of not paying for plasma donations for both ethical reasons and reasons of safety, but studies have found that relying on entirely voluntary plasma donation leads to shortages of immunoglobulin and forces member countries to import immunoglobulin from countries that do compensate donors.[41]
In Australia, blood donation is voluntary and therefore to cope with increasing demand and to reduce the shortages of locally produced immunoglobulin, several programs have been undertaken including adopting plasma for first time blood donors, better processes for donation, plasma donor centres and encouraging current blood donors to consider plasma only donation.[42]
Research
Experimental results from a small clinical trial in humans suggested protection against the progression of Alzheimer's Disease, but no such benefit was found in a subsequent phase III clinical trial.[43][44][45] In May 2020, the USA approved a phase three clinical trial on the efficacy and safety of high-concentration intravenous immune globulin therapy in severe COVID-19.[46]
References
- 1 2 3 4 5 6 7 8 9 10 "Immune Globulin". The American Society of Health-System Pharmacists. Archived from the original on 9 January 2017. Retrieved 8 January 2017.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 14 September 2020.
- 1 2 3 4 5 6 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 867–871. ISBN 9780857111562.
- ↑ Etzioni, Amos; Ochs, Hans D. (2014). Primary Immunodeficiency Disorders: A Historic and Scientific Perspective. Academic Press. pp. 283–284. ISBN 9780124115545. Archived from the original on 9 January 2017.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 ORANGE, J; HOSSNY, E; WEILER, C; BALLOW, M; BERGER, M; BONILLA, F; BUCKLEY, R; CHINEN, J; ELGAMAL, Y; MAZER, B (April 2006). "Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology". Journal of Allergy and Clinical Immunology (Review). 117 (4): S525–S553. doi:10.1016/j.jaci.2006.01.015. PMID 16580469. Archived from the original on 22 April 2020. Retrieved 26 January 2020.

- ↑ van Doorn, Pieter A.; Kuitwaard, Krista; Walgaard, Christa; van Koningsveld, Rinske; Ruts, Liselotte; Jacobs, Bart C. (16 April 2010). "IVIG Treatment and Prognosis in Guillain–Barré Syndrome". Journal of Clinical Immunology. 30 (S1): 74–78. doi:10.1007/s10875-010-9407-4. PMC 2883091. PMID 20396937.
- ↑ Hughes, R.A.C.; Wijdicks, E.F.M.; Barohn, R.; Benson, E.; Cornblath, D.R.; Hahn, A. F.; Meythaler, J.M.; Miller, R.G.; Sladky, J.T.; Stevens, J.C. (22 September 2003). "Practice parameter: Immunotherapy for Guillain–Barre syndrome: Report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 61 (6): 736–740. doi:10.1212/WNL.61.6.736. PMID 14504313.

- ↑ Pondrom, Sue (October 2014). "The IVIg Dilemma". The AJT Report. American Journal of Transplantation. 14 (10): 2195–6. doi:10.1111/ajt.12995. PMID 25231064.

- ↑ "CLINICAL GUIDELINES FOR IMMUNOGLOBULIN USE" (PDF). National Health Service. NHS. Archived (PDF) from the original on 29 May 2015. Retrieved 5 December 2015.
- ↑ "Eight guiding principals for safe, effective and appropriate use of IVIG" (PDF). American Association of Allergists and Immunologists. American Association of Allergists and Immunologists. Archived (PDF) from the original on 5 March 2016. Retrieved 5 December 2015.
- ↑ Anderson, David; Ali, Kaiser; Blanchette, Victor; Brouwers, Melissa; Couban, Stephen; Radmoor, Paula; Huebsch, Lothar; Hume, Heather; McLeod, Anne; Meyer, Ralph; Moltzan, Catherine; Nahirniak, Susan; Nantel, Stephen; Pineo, Graham; Rock, Gail (April 2007). "Guidelines on the Use of Intravenous Immune Globulin for Hematologic Conditions". Transfusion Medicine Reviews. 21 (2 Suppl 1): S9–S56. doi:10.1016/j.tmrv.2007.01.001. PMID 17397769.
- ↑ "Criteria for the Clinical Use of Intravenous Immunoglobulin in Australia | National Blood Authority". www.blood.gov.au. Archived from the original on 1 March 2020. Retrieved 14 November 2019.
- ↑ "Access to Intravenous Immunoglobulin (IVIg) | National Blood Authority". www.blood.gov.au. Archived from the original on 2 April 2020. Retrieved 14 November 2019.
- ↑ "Access to Subcutaneous Immunoglobulin (SCIg) | National Blood Authority". www.blood.gov.au. Archived from the original on 1 March 2020. Retrieved 14 November 2019.
- ↑ "HyQvia EPAR". European Medicines Agency (EMA). Archived from the original on 15 July 2020. Retrieved 14 July 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Hizentra EPAR". European Medicines Agency (EMA). Archived from the original on 1 August 2020. Retrieved 2 May 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Privigen EPAR". European Medicines Agency (EMA). Archived from the original on 15 July 2020. Retrieved 14 July 2020.
- ↑ "Kiovig EPAR". European Medicines Agency (EMA). Archived from the original on 17 July 2020. Retrieved 14 July 2020.
- 1 2 3 4 5 6 7 8 "Flebogamma DIF". European Medicines Agency (EMA). Archived from the original on 15 July 2020. Retrieved 15 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 4 5 6 "Immune Globulin". Dynamed Plus. EBSCO Health. Archived from the original on 8 December 2015. Retrieved 23 November 2015.(Subscription may be required or content may be available in libraries.)
- ↑ Daw, Z; Padmore, R; Neurath, D; Cober, N; Tokessy, M; Desjardins, D; Olberg, B; Tinmouth, A; Giulivi, A (August 2008). "Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis". Transfusion. 48 (8): 1598–601. doi:10.1111/j.1537-2995.2008.01721.x. PMID 18466176.
- ↑ "Hyqvia (immune globulin 10 percent- human with recombinant human hyaluronidase) kit". DailyMed. Archived from the original on 5 August 2020. Retrieved 1 May 2020.
- ↑ Lichtiger, Lichtiger. "Laboratory Serologic Problems Associated with Administration of Intravenous IgG". Current Issues in Transfusion Medicine. he University of Texas M. D. Anderson Cancer Center. Archived from the original on 5 March 2016. Retrieved 23 November 2015.
- 1 2 3 4 5 6 7 8 Skoda-Smith, Suzanne; Torgerson, Troy; Ochs, Hans (16 December 2009). "Subcutaneous immunoglobulin replacement therapy in the treatment of patients with primary immunodeficiency disease". Therapeutics and Clinical Risk Management. 2010:6: 1–10. doi:10.2147/tcrm.s4353. PMC 2817783. PMID 20169031.
- ↑ Misbah S, Sturzenegger MH, Borte M, Shapiro RS, Wasserman RL, Berger M, Ochs HD (December 2009). "Subcutaneous immunoglobulin: opportunities and outlook". Clin. Exp. Immunol. 158 Suppl 1: 51–9. doi:10.1111/j.1365-2249.2009.04027.x. PMC 2801034. PMID 19883424.
- ↑ Powell, Lynne. "Re: A Message from CSL Behring to Current Vivaglobin Patients in the United States" (PDF). Primary Immune Foundation. CSL Behring. Archived (PDF) from the original on 22 December 2015. Retrieved 24 November 2015.
- ↑ Rajabally, Yusuf A. (April 2022). "Immunoglobulin and Monoclonal Antibody Therapies in Guillain-Barré Syndrome". Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 19 (3): 885–896. doi:10.1007/s13311-022-01253-4. ISSN 1878-7479. Retrieved 6 May 2023.
- ↑ Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE (1998). "Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin". Science. 282 (5388): 490–3. Bibcode:1998Sci...282..490V. doi:10.1126/science.282.5388.490. PMID 9774279.
- 1 2 3 4 Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB (2015). "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity". J Autoimmun. 57 (6): 1–13. doi:10.1016/j.jaut.2014.12.002. PMC 4340844. PMID 25578468.
- ↑ Gern JE (August 2002). "Antiinflammatory Activity of IVIG Mediated through the Inhibitory FC Receptor". Pediatrics. 110 (2): 467–8. doi:10.1542/peds.110.2.S1.467-b (inactive 15 March 2020). Archived from the original on 11 June 2008.
{{cite journal}}: CS1 maint: DOI inactive as of March 2020 (link) - ↑ Nimmerjahn F, Ravetch JV (January 2007). "The antiinflammatory activity of IgG: the intravenous IgG paradox". J. Exp. Med. 204 (1): 11–5. doi:10.1084/jem.20061788. PMC 2118416. PMID 17227911.
- ↑ Clynes R (January 2005). "Immune complexes as therapy for autoimmunity". J. Clin. Invest. 115 (1): 25–7. doi:10.1172/JCI23994. PMC 539209. PMID 15630438.
- ↑ Siragam V, Crow AR, Brinc D, Song S, Freedman J, Lazarus AH (June 2006). "Intravenous immunoglobulin ameliorates ITP via activating Fc gamma receptors on dendritic cells". Nat. Med. 12 (6): 688–92. doi:10.1038/nm1416. PMID 16715090.
- ↑ Bayry J, Thirion M, Misra N, et al. (October 2003). "Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases". Neurol. Sci. 24 Suppl 4: S217–21. doi:10.1007/s10072-003-0081-7. PMID 14598046.
- ↑ Janke AD, Yong VW (April 2006). "Impact of IVIg on the interaction between activated T cells and microglia". Neurol. Res. 28 (3): 270–4. doi:10.1179/016164106X98143. PMID 16687052.
- ↑ "Eight Guiding Principles for Effective Use of IVIG for Patients with Primary Immunodeficiency" (PDF). American Association of Allergists and Immunologists. Archived (PDF) from the original on 5 March 2016. Retrieved 23 November 2015.
- ↑ "Hizentra". U.S. Food and Drug Administration (FDA). 15 March 2018. Archived from the original on 1 April 2020. Retrieved 3 May 2020.
- ↑ Pierce, L. Ross (14 March 2018). Summary Basis for Regulatory Action - Hizentra (PDF) (Report). Review Office Signatory Authority Tejashri Purohit-Sheth. U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 20 March 2018. Retrieved 20 March 2018.
- ↑ Berman, Keith. "SCIG: New Therapeutic Uses Beyond PI?" (PDF). IG Living (February–March 2015): 28–32. Archived (PDF) from the original on 27 October 2015. Retrieved 23 November 2015.
- 1 2 "An EU-wide overview of the market of blood, blood components and plasma derivatives focusing on their availability for patients Creative Ceutical Report, revised by the Commission to include stakeholders' comments" (PDF). Creative Ceutical & EU commission. Archived (PDF) from the original on 22 December 2015. Retrieved 7 December 2015.
- ↑ "transfusion.com.au". transfusion.com.au. Archived from the original on 4 September 2019. Retrieved 14 November 2019.
- ↑ Andrew Pollack (17 July 2012). "Small Trial Hints Drug Can Slow Alzheimer's". New York Times. Archived from the original on 19 July 2012. Retrieved 19 July 2012.
- ↑ "Experimental Alzheimer's drug Gammagard may stall memory decline, small study suggests". CBS News. 17 July 2012. Archived from the original on 19 July 2012. Retrieved 19 July 2012.
- ↑ Another Alzheimer's Drug Fails Phase III Trial Archived 2013-06-07 at the Wayback Machine
- ↑ "FDA Approves Octapharma USA Investigational New Drug Application for Severe COVID-19 Patients". www.businesswire.com. 20 May 2020. Archived from the original on 21 June 2020. Retrieved 28 May 2020.
External links
| Identifiers: |
|---|
- "Immune globulin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 31 January 2020. Retrieved 31 January 2020.