Proton-pump inhibitors
| Proton-pump inhibitors | |
|---|---|
| Drug class | |
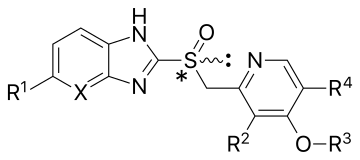 General structure of a proton-pump inhibitor | |
| Names | |
| Stem | -pazole[1] |
| Clinical data | |
| Uses | Peptic ulcers, gastroesophageal reflux disease (GERD), H. pylori infection[2] |
| Common types | Omeprazole, pantoprazole, rabeprazole, lansoprazole, esomeprazole, dexlansoprazole[3] |
| Mechanism of action | Enzyme inhibitor[3] |
| Biological target | H+/K+ ATPase[2] |
| External links | |
| Drugs.com | Drug Classes |
| WebMD | MedicineNet |
Proton-pump inhibitors (PPIs) are a class of medications used for peptic ulcers, gastroesophageal reflux disease (GERD), and H. pylori infection.[2] Common agents in this class include omeprazole, pantoprazole, rabeprazole, lansoprazole, esomeprazole, and dexlansoprazole.[3] There is no clear evidence that one agent works better than another.[4][5] They can be taken by mouth or given intravenously.[2]
PPIs are generally safe.[3] Common side effects may include headache, an upset stomach, and a change in taste.[2][3] Serious side effects may include kidney failure, osteoporosis, low magnesium, and Clostridium difficile-associated diarrhea.[2][3] While concerns have been raised about an interaction with clopidogrel, the significance if any is unclear.[6]
They work by decreasing stomach acid production.[2] They do so by blocking the H+/K+ ATPase.[2] They are the strongest inhibitors of acid secretion available.[7] This group decreases stomach acid more than H2-receptor antagonists.[8]
The first medically useful PPI, omeprazole, was made in 1979.[9] They are among the most widely used medications.[2] One agent within the class, omeprazole, is on the World Health Organization's List of Essential Medicines.[10] Some are available as generic medication and are relatively inexpensive.[2]
Types
Proton pump inhibitors:
- Omeprazole (Over-the-counter drug (OTC) and Rx-only in the US)[11]
- Lansoprazole (OTC and Rx-only in the US)[12]
- Dexlansoprazole[12]
- Esomeprazole (OTC and Rx-only in the US and Australia)[11]
- Pantoprazole[13]
- Rabeprazole[14]
- Ilaprazole (not FDA-approved as of July 2019)
Medical uses
Medical uses include:
- Dyspepsia[15][16]
- Peptic ulcer disease including after endoscopic treatment for bleeding[17]
- As part of Helicobacter pylori eradication therapy[18]
- Gastroesophageal reflux disease (GERD or GORD) including symptomatic endoscopy-negative reflux disease[19] and associated laryngopharyngeal reflux causing laryngitis[20] and chronic cough.[21] Use in babies is not recommended due to lack of benefit and evidence of harm.[22]
- Barrett's esophagus[23] Although they reduce the incidence of esophageal adenocarcinoma in Barrett's oesophagus,[23] they do not change the length affected.[24]
- Eosinophilic esophagitis[25]
- Stress gastritis and ulcer prevention in critical care[26]
- Gastrinomas and other conditions that cause hypersecretion of acid including Zollinger–Ellison syndrome (often 2–3x the regular dose is required)[27]
In children under the age of one who have fussiness or regurgitation with feeds, they do not result in benefits[28]
Dosage
Professional organizations recommend that people take the lowest effective PPI dose to achieve the desired result when used to treat gastroesophageal reflux disease long-term.[29][30][31] In the United States, the Food and Drug Administration (FDA) has advised that no more than three 14-day treatment courses should be used in one year.[32][33]
Indications for stopping
PPIs are often used longer than necessary. In about half of people who are hospitalized or seen at a primary care clinic there is no documented reason for their long-term use of PPIs.[34] Some researchers believe that, given the little evidence of long-term effectiveness, the cost of the medication and the potential for harm means that clinicians should consider stopping PPIs in many people.[35]
After four weeks, if symptoms have resolved, the PPI may be stopped in those who were using them for heartburn, gastroesophageal reflux disease, or inflammation of the esophagus if these last two were not severe.[34] Stopping is not recommended in those with Barrett's esophagus or a bleeding stomach ulcer.[34] Stopping may be carried out by first decreasing the amount of medication taken or having the person take the medication only when symptoms are present.[36]
Side effects
In general, proton pump inhibitors are well tolerated, and the incidence of short-term adverse effects is relatively low. The range and occurrence of side effects are similar for all of the PPIs, though they have been reported more frequently with omeprazole. This may be due to its longer availability and, hence, use.
Common side effects include headache, nausea, diarrhea, abdominal pain, fatigue, and dizziness.[37] Infrequent side effects include rash, itch, flatulence, constipation, anxiety, and depression. Also infrequently, PPI use may be associated with occurrence of myopathies, including the serious reaction rhabdomyolysis.[38]
Long-term use of PPIs requires assessment of the balance of the benefits and risks of the therapy.[39][40][41] Various negative outcomes have been associated with long-term PPI use in several primary reports, but reviews assess the overall quality of evidence in these studies as "low" or "very low".[40] They describe inadequate evidence to establish causal relationships between PPI therapy and many of the proposed associations, due to study design and small estimates of effect size.[41] Benefits outweigh risks when PPIs are used appropriately, but when used inappropriately, modest risks become important.[40] They recommend that PPIs should be used at the lowest effective dose in people with a proven indication, but discourage dose escalation and continued chronic therapy in people unresponsive to initial empiric therapy.[41]
Nutritional
Gastric acid is important for breakdown of food and release of micronutrients, and some studies have shown possibilities for interference with absorption of iron, calcium, magnesium, and vitamin B12.[42] With regard to iron and vitamin B12, the data are weak and several confounding factors have been identified.[39][42]
Low levels of magnesium can be found in people on PPI therapy and these can be reversed when they are switched to H2-receptor antagonist medications.[39][43][33]
High dose or long-term use of PPIs carries a possible increased risk of bone fractures which was not found with short-term, low dose use; the FDA included a warning regarding this on PPI drug labels in 2010.[32]
Gastrointestinal
Some studies have shown a correlation between use of PPIs and Clostridioides difficile infection. While the data are contradictory and controversial, the FDA had sufficient concern to include a warning about this adverse effect on the label of PPI medications.[39] Concerns have also been raised about spontaneous bacterial peritonitis in older people taking PPIs and in people with irritable bowel syndrome taking PPIs; both types of infections arise in these populations due to underlying conditions and it is not clear if this is a class effect of PPIs.[39] PPIs may predispose an individual to developing small intestinal bacterial overgrowth or fungal overgrowth.[44][45]
Long-term use of PPIs is associated with the development of benign polyps from fundic glands (which is distinct from fundic gland polyposis); these polyps do not cause cancer and resolve when PPIs are discontinued.[39] There is concern that use of PPIs may mask gastric cancers or other serious gastric problems.[39]
PPI use has also been associated with the development of microscopic colitis.[46]
There is also evidence that PPI use alters the composition of the bacterial populations inhabiting the gut.[47] Although the mechanisms by which PPIs cause these changes are yet to be determined they may have a role in the increased risk of bacterial infections with PPI use. These infections can include Helicobacter pylori due to this species not favouring an acid environment, leading an increased risk of ulcers and Gastric cancer risk in genetically susceptible patients.[48]
PPI use in subjects who have received attempted H. pylori eradication may also be associated with an increased risk of gastric cancer.[49] The validity and robustness of this finding, with the lack of causality, have led to this association being questioned.[50] It is recommended that long-term PPIs should be used judiciously after considering individual's risk–benefit profile, particularly among those with history of H. pylori infection, and that further, well-designed, prospective studies are needed.[51]
Cardiovascular
Associations of PPI use and cardiovascular events have also been widely studied but clear conclusions have not been made as these relative risks are confounded by other factors.[52][53] PPIs are commonly used in people with cardiovascular disease for gastric protection when aspirin is given for its antiplatelet actions.[52][54] An interaction between PPIs and the metabolism of the platelet inhibitor clopidogrel is known and this drug is also often used in people with cardiac disease.[55][56][31]
One suggested mechanism for cardiovascular effects is because PPIs bind and inhibit dimethylargininase, the enzyme that degrades asymmetric dimethylarginine (ADMA), resulting in higher ADMA levels and a decrease in bioavailable nitric oxide.[57]
Other
Associations have been shown between PPI use and an increased risk of pneumonia, particularly in the 30 days after starting therapy, where it was found to be 50% higher in community use.[58][59] Other very weak associations of PPI use have been found, such as with chronic kidney disease[60][61][62][31][63][64] and dementia.[65][40][66] As these results were derived from observational studies, it remains uncertain whether such associations are causal relationships.[40][41][67]
Mechanism of action
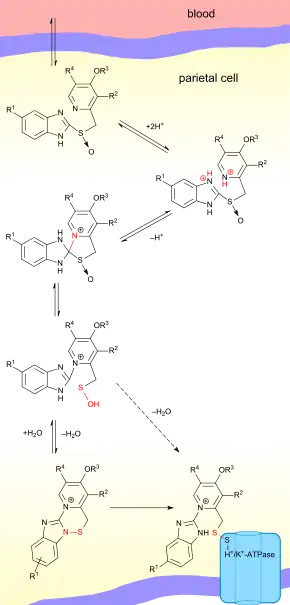
Proton pump inhibitors act by irreversibly blocking the hydrogen/potassium adenosine triphosphatase enzyme system (the H+/K+ ATPase, or, more commonly, the gastric proton pump) of the gastric parietal cells.[68] The proton pump is the terminal stage in gastric acid secretion, being directly responsible for secreting H+ ions into the gastric lumen, making it an ideal target for inhibiting acid secretion.
Targeting the terminal step in acid production, as well as the irreversible nature of the inhibition, results in a class of medications that are significantly more effective than H2 antagonists and reduce gastric acid secretion by up to 99%.[7]
Decreasing the acid in the stomach can aid the healing of duodenal ulcers and reduce the pain from indigestion and heartburn. However, stomach acids are needed to digest proteins, vitamin B12, calcium, and other nutrients, and too little stomach acid causes the condition hypochlorhydria.
The PPIs are given in an inactive form, which is neutrally charged (lipophilic) and readily crosses cell membranes into intracellular compartments (like the parietal cell canaliculus) with acidic environments. In an acid environment, the inactive drug is protonated and rearranges into its active form. As described above, the active form will covalently and irreversibly bind to the gastric proton pump, deactivating it.
Pharmacokinetics
The rate of omeprazole absorption is decreased by concomitant food intake.[69] In addition, the absorption of lansoprazole and esomeprazole is decreased and delayed by food. It has been reported, however, that these pharmacokinetic effects have no significant impact on efficacy.[70][71]
PPIs have a half-life in human blood plasma of only 60–90 minutes, but because they covalently bind to the pump, the half-life of their inhibition of gastric acid secretion lasts an estimated 24 hours. Dissociation of the inhibitory complex is probably due to the effect of the endogenous antioxidant glutathione which leads to the release of omeprazole sulfide and reactivation of the enzyme.[72][73]
History
PPIs were developed in the 1980s, with omeprazole being launched in 1988. Most of these medications are benzimidazole derivatives, related to omeprazole, but imidazopyridine derivatives such as tenatoprazole have also been developed.[7] Potassium-competitive inhibitors such as revaprazan reversibly block the potassium-binding site of the proton pump, acting more quickly, but are not available in most countries.[74]
Society and culture
Cost
In British Columbia, Canada the cost of the PPIs varies significantly from CA$0.13 to CA$2.38 per dose[75] while all agents in the class appear more or less equally effective.[4][5]
Regulatory approval
A comparative table of FDA-approved indications for PPIs is shown below.
| Indication | Omeprazole | Esomeprazole | Lansoprazole | Dexlansoprazole | Pantoprazole | Rabeprazole |
|---|---|---|---|---|---|---|
| Gastroesophageal reflux disease | ||||||
| Erosive esophagitis-healing | Yes | Yes | Yes | Yes | Yes | Yes |
| Erosive esophagitis-maintenance | Yes | Yes | Yes | Yes | Yes | Yes |
| Nonerosive reflux disease | Yes | Yes | Yes | Yes | No | Yes |
| Peptic ulcer disease | ||||||
| Duodenal ulcer-healing | Yes | No | Yes | No | No | Yes |
| Duodenal ulcer-maintenance | No | No | Yes | No | No | No |
| Gastric ulcer-healing | Yes | No | Yes | No | No | No |
| NSAID induced ulcer-healing | No | No | Yes | No | No | No |
| NSAID induced ulcer-prophylaxis | No | Yes | Yes | No | No | No |
| Zollinger-Ellison syndrome | Yes | Yes | Yes | No | Yes | Yes |
| Treatment of Helicobacter pylori | ||||||
| Dual therapy | Yes | No | Yes | No | No | No |
| Triple therapy | Yes | Yes | Yes | No | No | Yes |
References
- ↑ "Generic Name Stems - Drug Information Portal - U.S. National Library of Medicine". druginfo.nlm.nih.gov. Archived from the original on 30 March 2021. Retrieved 13 March 2021.
- 1 2 3 4 5 6 7 8 9 10 Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. p. 194. ISBN 978-0-7020-7442-4. Archived from the original on 2021-05-22. Retrieved 2021-11-09.
- 1 2 3 4 5 6 "List of Proton Pump Inhibitors + Uses, Side Effects". Drugs.com. Archived from the original on 29 August 2021. Retrieved 12 March 2021.
- 1 2 "[99] Comparative effectiveness of proton pump inhibitors". Therapeutics Letter. 28 June 2016. ISSN 2369-8691. Archived from the original on 9 July 2016. Retrieved 14 July 2016.
- 1 2 Dean, Laura (1 October 2010). Comparing Proton Pump Inhibitors. PubMed Health. National Center for Biotechnology Information (US). Archived from the original on 8 March 2018. Retrieved 16 July 2016.
- ↑ "What is the clinical relevance of the clopidogrel/proton pump inhibitor (PPI) interaction?". 2019. Archived from the original on 27 September 2020. Retrieved 13 March 2021.
- 1 2 3 Sachs, G.; Shin, J. M.; Howden, C. W. (2006). "Review article: The clinical pharmacology of proton pump inhibitors". Alimentary Pharmacology and Therapeutics. 23: 2–8. doi:10.1111/j.1365-2036.2006.02943.x. PMID 16700898. S2CID 30413194.
- ↑ Hauser, Stephen C.; Pardi, Darrell S.; Poterucha, John J. (2005). Mayo Clinic Gastroenterology and Hepatology Board Review. CRC Press. p. 18. ISBN 978-0-203-50274-7. Archived from the original on 2021-08-29. Retrieved 2021-03-13.
- ↑ Olbe, Lars (1999). Proton Pump Inhibitors. Springer Science & Business Media. p. 17. ISBN 978-3-7643-5897-6. Archived from the original on 2021-08-29. Retrieved 2021-03-13.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "Omeprazole and Esomeprazole Omeprazole at the Wayback Machine (archived 2018-05-09)". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- 1 2 "Lansoprazole, Dexlansoprazole Lansoprazole at the Wayback Machine (archived 2018-05-09)". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ "Pantoprazole Pantoprazole at the Wayback Machine (archived 2018-05-09)". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ "Rabeprazole Rabeprazole at the Wayback Machine (archived 2017-10-13)". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ Zajac, P; Holbrook, A; Super, ME; Vogt, M (March–April 2013). "An overview: Current clinical guidelines for the evaluation, diagnosis, treatment, and management of dyspepsia". Osteopathic Family Physician. 5 (2): 79–85. doi:10.1016/j.osfp.2012.10.005.
- ↑ Wang WH, Huang JQ, Zheng GF, Xia HH, Wong WM, Liu XG, et al. (2007). "Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials". Clinical Gastroenterology and Hepatology. 5 (2): 178–85, quiz 140. doi:10.1016/j.cgh.2006.09.012. PMID 17174612.
- ↑ Sachar H, Vaidya K, Laine L (2014). "Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: a systematic review and meta-analysis". JAMA Internal Medicine. 174 (11): 1755–62. doi:10.1001/jamainternmed.2014.4056. PMC 4415726. PMID 25201154.
- ↑ Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, et al. (2013). "Optimum duration of regimens for Helicobacter pylori eradication". Cochrane Database of Systematic Reviews. 12 (12): CD008337. doi:10.1002/14651858.CD008337.pub2. PMID 24338763.
- ↑ Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME (2013). "Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease". Cochrane Database of Systematic Reviews. 5 (5): CD002095. doi:10.1002/14651858.CD002095.pub5. PMC 7066537. PMID 23728637.
- ↑ Qadeer MA, Phillips CO, Lopez AR, Steward DL, Noordzij JP, Wo JM, et al. (2006). "Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials". The American Journal of Gastroenterology. 101 (11): 2646–54. PMID 17037995. Archived from the original on 2020-01-14. Retrieved 2020-01-14.
- ↑ Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA (2006). "Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux". The BMJ. 332 (7532): 11–7. doi:10.1136/bmj.38677.559005.55. PMC 1325125. PMID 16330475.
- ↑ Lassalle, M; Zureik, M; Dray-Spira, R (14 August 2023). "Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children". JAMA pediatrics. doi:10.1001/jamapediatrics.2023.2900. PMID 37578761.
- 1 2 Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB (2014). "Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis". Gut. 63 (8): 1229–37. doi:10.1136/gutjnl-2013-305997. PMC 4199831. PMID 24221456.
- ↑ Cooper, B. T.; Chapman, W.; Neumann, C. S.; Gearty, J. C. (2006). "Continuous treatment of Barrett's oesophagus patients with proton pump inhibitors up to 13 years: Observations on regression and cancer incidence". Alimentary Pharmacology and Therapeutics. 23 (6): 727–33. doi:10.1111/j.1365-2036.2006.02825.x. PMID 16556174. S2CID 6969621.
- ↑ Lucendo AJ, Arias Á, Molina-Infante J (2015). "Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histological Remission in Patients with Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis". Clinical Gastroenterology and Hepatology. 14 (1): 13–22.e1. doi:10.1016/j.cgh.2015.07.041. PMID 26247167.
- ↑ Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ (2013). "Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis". Critical Care Medicine. 41 (3): 693–705. doi:10.1097/CCM.0b013e3182758734. PMID 23318494. S2CID 8138473.
- ↑ Epelboym I, Mazeh H (2014). "Zollinger-Ellison syndrome: classical considerations and current controversies". Oncologist. 19 (1): 44–50. doi:10.1634/theoncologist.2013-0369. PMC 3903066. PMID 24319020.
- ↑ Ambartsumyan, Armen (14 May 2023). "#340 Crying babies: Can proton pump inhibitors help? (Free)". CFPCLearn. Archived from the original on 1 July 2023. Retrieved 13 June 2023.
- ↑ "Five Things Physicians and Patients Should Question". American Gastroenterological Association. Archived from the original on 2015-03-29. Retrieved 2013-03-01.
- ↑ Kahrilas, Peter J.; Shaheen, Nicholas J.; Vaezi, Michael F.; Hiltz, SW; Black, E; Modlin, IM; Johnson, SP; Allen, J; Brill, JV (2008). "American Gastroenterological Association Medical Position Statement on the Management of Gastroesophageal Reflux Disease". Gastroenterology. 135 (4): 1383–1391, 1391.e1–5. doi:10.1053/j.gastro.2008.08.045. PMID 18789939.
- 1 2 3 Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z (30 May 2019). "Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study". BMJ. 365: l1580. doi:10.1136/bmj.l1580. ISSN 0959-8138. PMC 6538974. PMID 31147311.
Taking PPIs is associated with a small excess of cause specific mortality including death due to cardiovascular disease, chronic kidney disease, and upper gastrointestinal cancer. The burden was also observed in patients without an indication for PPI use.
- Lay summary in: "Heartburn drugs linked to fatal heart and kidney disease, stomach cancer". Washington University School of Medicine. 30 May 2019.
- 1 2 "FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors". U.S. Food and Drug Administration (FDA). 23 March 2011. Archived from the original on 20 March 2020. Retrieved 23 August 2015.
- 1 2 "Low magnesium levels can be associated with long-term use of PPIs". U.S. Food and Drug Administration (FDA). 17 November 2009. Archived from the original on 12 May 2020. Retrieved 23 February 2020.
- 1 2 3 Farrell, B; Pottie, K; Thompson, W; Boghossian, T; Pizzola, L; Rashid, FJ; Rojas-Fernandez, C; Walsh, K; Welch, V; Moayyedi, P (May 2017). "Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline". Canadian Family Physician. 63 (5): 354–364. PMC 5429051. PMID 28500192.
- ↑ "Canadian Cardiovascular Society and Choosing Wisely Canada: The Road to Creating a List of Five Things Physicians and Patients Should Question". Canadian Journal of Cardiology. 30 (8): 949–955. August 2014. doi:10.1016/j.cjca.2014.06.010. ISSN 0828-282X.
- ↑ "[111] Deprescribing Proton Pump Inhibitors". Therapeutics Initiative. 26 June 2018. Archived from the original on 13 April 2019. Retrieved 27 June 2018.
- ↑ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Clark, DW; Strandell J (June 2006). "Myopathy including polymyositis: a likely class adverse effect of proton pump inhibitors?". European Journal of Clinical Pharmacology. 62 (6): 473–479. doi:10.1007/s00228-006-0131-1. PMID 16758264. S2CID 33139851.
- 1 2 3 4 5 6 7 Corleto VD, Festa S, Di Giulio E, Annibale B (February 2014). "Proton pump inhibitor therapy and potential long-term harm". Current Opinion in Endocrinology, Diabetes and Obesity. 21 (1): 3–8. doi:10.1097/MED.0000000000000031. PMID 24310148. S2CID 205791135.
- 1 2 3 4 5 Freedberg DE, Kim LS, Yang YX (2017). "The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association". Gastroenterology. 152 (4): 706–715. doi:10.1053/j.gastro.2017.01.031. PMID 28257716.
Conclusions:Baseline differences between PPI users and non-users make it challenging to study potential PPI adverse effects retrospectively. Despite a large number of studies, the overall quality of evidence for PPI adverse effects is low to very low. When PPIs are appropriately prescribed, their benefits are likely to outweigh their risks. When PPIs are inappropriately prescribed, modest risks become important because there is no potential benefit. There is currently insufficient evidence to recommend specific strategies for mitigating PPI adverse effects.
- 1 2 3 4 Vaezi MF, Yang YX, Howden CW (2017). "Complications of Proton Pump Inhibitor Therapy". Gastroenterology. 153 (1): 35–48. doi:10.1053/j.gastro.2017.04.047. PMID 28528705.
In turn, this has caused unnecessary concern among patients and prescribers. The benefits of PPI therapy for appropriate indications need to be considered, along with the likelihood of the proposed risks. Patients with a proven indication for a PPI should continue to receive it in the lowest effective dose. PPI dose escalation and continued chronic therapy in those unresponsive to initial empiric therapy is discouraged.
- 1 2 Ito T, Jensen RT (2010). "Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium". Current Gastroenterology Reports. 12 (6): 448–57. doi:10.1007/s11894-010-0141-0. PMC 2974811. PMID 20882439.
- ↑ Park CH, Kim EH, Roh YH, Kim HY, Lee SK (2014). "The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis". PLOS ONE. 9 (11): e112558. Bibcode:2014PLoSO...9k2558P. doi:10.1371/journal.pone.0112558. PMC 4230950. PMID 25394217.
- ↑ Fujimori S (2015). "What are the effects of proton pump inhibitors on the small intestine?". World J. Gastroenterol. 21 (22): 6817–9. doi:10.3748/wjg.v21.i22.6817. PMC 4462721. PMID 26078557.
Generally, proton-pump inhibitors (PPIs) have great benefit for patients with acid related disease with less frequently occurring side effects. According to a recent report, PPIs provoke dysbiosis of the small intestinal bacterial flora, exacerbating nonsteroidal anti-inflammatory drug-induced small intestinal injury. Several meta-analyses and systematic reviews have reported that patients treated with PPIs, as well as post-gastrectomy patients, have a higher frequency of small intestinal bacterial overgrowth (SIBO) compared to patients who lack the aforementioned conditions. Furthermore, there is insufficient evidence that these conditions induce Clostridium difficile infection. At this time, PPI-induced dysbiosis is considered a type of SIBO.
- ↑ Erdogan A, Rao SS (April 2015). "Small intestinal fungal overgrowth". Curr Gastroenterol Rep. 17 (4): 16. doi:10.1007/s11894-015-0436-2. PMID 25786900. S2CID 3098136.
Small intestinal fungal overgrowth (SIFO) is characterized by the presence of excessive number of fungal organisms in the small intestine associated with gastrointestinal (GI) symptoms. Candidiasis is known to cause GI symptoms particularly in immunocompromised patients or those receiving steroids or antibiotics. However, only recently, there is emerging literature that an overgrowth of fungus in the small intestine of non-immunocompromised subjects may cause unexplained GI symptoms. Two recent studies showed that 26% (24/94) and 25.3% (38/150) of a series of patients with unexplained GI symptoms had SIFO. The most common symptoms observed in these patients were belching, bloating, indigestion, nausea, diarrhea, and gas. The underlying mechanism(s) that predisposes to SIFO is unclear but small intestinal dysmotility and use of proton pump inhibitors has been implicated. However, further studies are needed; both to confirm these observations and to examine the clinical relevance of fungal overgrowth, both in healthy subjects and in patients with otherwise unexplained GI symptoms.
- ↑ Münch A, Aust D, Bohr J, Bonderup O, Fernández Bañares F, Hjortswang H, et al. (2012). "Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group". Journal of Crohn's and Colitis. 6 (9): 932–45. doi:10.1016/j.crohns.2012.05.014. PMID 22704658.
- ↑ Jackson, Matthew A.; Goodrich, Julia K.; Maxan, Maria-Emanuela; Freedberg, Daniel E.; Abrams, Julian A.; Poole, Angela C.; Sutter, Jessica L.; Welter, Daphne; Ley, Ruth E. (2015-12-30). "Proton pump inhibitors alter the composition of the gut microbiota". Gut. 65 (5): gutjnl–2015–310861. doi:10.1136/gutjnl-2015-310861. ISSN 1468-3288. PMC 4853574. PMID 26719299.
- ↑ Hagiwara T, Mukaisho K, Nakayama T, Hattori T, Sugihara H. Proton pump inhibitors and helicobacter pylori-associated pathogenesis. Asian Pac J Cancer Prev 2015; 16: 1315-1319 [DOI: 10.7314/APJCP.2015.16.4.1315]
- ↑ Cheung KS, Chan EW, Wong AY, Chen L, Wong IC, Leung WK (January 2018). "Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study". Gut. 67 (1): 28–35. doi:10.1136/gutjnl-2017-314605. ISSN 0017-5749. PMID 29089382.
- ↑ Leontiadis, GI; Veldhuyzen Van Zanten, S; Hookey, L; Armstrong, D; Jones, N; Moayyedi, P (December 2018). "Canadian Association of Gastroenterology Statement on the Putative Link Between Proton Pump Inhibitor Treatment and Gastric Cancer after Helicobacter pylori Eradication". Journal of the Canadian Association of Gastroenterology. 1 (4): 155–158. doi:10.1093/jcag/gwy040. PMC 6542241. PMID 31294357.
- ↑ Cheung KS, Leung WK (January 2019). "Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence". Therapeutic Advances in Gastroenterology. 12: 175628481983451. doi:10.1177/1756284819834511. ISSN 1756-2848. PMC 6415482. PMID 30886648.
- 1 2 Agewall S, Cattaneo M, Collet JP, Andreotti F, Lip GY, Verheugt FW, et al. (2013). "Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy". European Heart Journal. 34 (23): 1708–13, 1713a–1713b. doi:10.1093/eurheartj/eht042. PMID 23425521.
- ↑ Melloni C, Washam JB, Jones WS, Halim SA, Hasselblad V, Mayer SB, et al. (2015). "Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review". Circulation: Cardiovascular Quality and Outcomes. 8 (1): 47–55. doi:10.1161/CIRCOUTCOMES.114.001177. PMC 6143138. PMID 25587094.
- ↑ Kwok CS, Nijjar RS, Loke YK (2011). "Effects of proton pump inhibitors on adverse gastrointestinal events in patients receiving clopidogrel: systematic review and meta-analysis". Drug Safety. 34 (1): 47–57. doi:10.2165/11584750-000000000-00000. PMID 21047145. S2CID 21231797.
- ↑ Focks JJ, Brouwer MA, van Oijen MG, Lanas A, Bhatt DL, Verheugt FW (2013). "Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome- a systematic review". Heart. 99 (8): 520–7. doi:10.1136/heartjnl-2012-302371. PMID 22851683. S2CID 23689175.
- ↑ Cardoso RN, Benjo AM, DiNicolantonio JJ, Garcia DC, Macedo FY, El-Hayek G, et al. (2015). "Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis". Open Heart. 2 (1): e000248. doi:10.1136/openhrt-2015-000248. PMC 4488889. PMID 26196021.
- ↑ Schepers E, Speer T, Bode-Böger SM, Fliser D, Kielstein JT (2014). "Dimethylarginines ADMA and SDMA: the real water-soluble small toxins?". Seminars in Nephrology. 34 (2): 97–105. doi:10.1016/j.semnephrol.2014.02.003. PMID 24780466.
It also seems to be the pathophysiological link between the use of proton pump inhibitors and increased cardiovascular event rate because these medications bind and inhibit DDAH, the enzyme that degrades ADMA, which results in higher ADMA levels and a decrease in bioavailable NO.
- ↑ Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA (2015). "Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis". PLOS ONE. 10 (6): e0128004. Bibcode:2015PLoSO..1028004L. doi:10.1371/journal.pone.0128004. PMC 4456166. PMID 26042842.
- ↑ Eom, CS; Jeon, CY; Lim, JW; Cho, EG; Park, SM; Lee, KS (22 February 2011). "Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis". CMAJ : Canadian Medical Association Journal. 183 (3): 310–9. doi:10.1503/cmaj.092129. PMC 3042441. PMID 21173070.
- ↑ Hussain, Salman; Singh, Ambrish; Habib, Anwar; Najmi, Abul Kalam (2019). "Proton pump inhibitors use and risk of chronic kidney disease: Evidence-based meta-analysis of observational studies". Clinical Epidemiology and Global Health. 7: 46–52. doi:10.1016/j.cegh.2017.12.008.
- ↑ Lazarus, Benjamin; Chen, Yuan; Wilson, Francis P.; Sang, Yingying; Chang, Alex R.; Coresh, Josef; Grams, Morgan E. (2016-02-01). "Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease". JAMA Internal Medicine. American Medical Association (AMA). 176 (2): 238–46. doi:10.1001/jamainternmed.2015.7193. ISSN 2168-6106. PMC 4772730. PMID 26752337.
- ↑ Xie, Yan; Bowe, Benjamin; Li, Tingting; Xian, Hong; Yan, Yan; Al-Aly, Ziyad (2017). "Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury". Kidney International. Elsevier BV. 91 (6): 1482–1494. doi:10.1016/j.kint.2016.12.021. ISSN 0085-2538. PMID 28237709.
- ↑ Moledina, Dennis G.; Perazella, Mark A. (2016-04-14). "Proton Pump Inhibitors and CKD". Journal of the American Society of Nephrology. American Society of Nephrology (ASN). 27 (10): 2926–2928. doi:10.1681/asn.2016020192. ISSN 1046-6673. PMC 5042680. PMID 27080978.
- ↑ Xie, Yan; Bowe, Benjamin; Li, Tingting; Xian, Hong; Balasubramanian, Sumitra; Al-Aly, Ziyad (2016-04-14). "Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD". Journal of the American Society of Nephrology. American Society of Nephrology (ASN). 27 (10): 3153–3163. doi:10.1681/asn.2015121377. ISSN 1046-6673. PMC 5042677. PMID 27080976.
- ↑ Salman Hussain, Ambrish Singh et al. No association between proton pump inhibitors use and risk of dementia: Evidence from a meta-analysis. J Gastroenterol Hepatol. https://doi.org/10.1111/jgh.14789 No association between proton pump inhibitor use and risk of dementia: Evidence from a meta‐analysis - Hussain - 2020 - Journal of Gastroenterology and Hepatology - Wiley Online Library at the Wayback Machine (archived 2021-08-29)
- ↑ Schnoll-Sussman F, Katz PO (2017). "Clinical Implications of Emerging Data on the Safety of Proton Pump Inhibitors". Curr Treat Options Gastroenterol. 15 (1): 1–9. doi:10.1007/s11938-017-0115-5. PMID 28130652. S2CID 24718665.
The methodology of these studies allows us to find an association with these events but does not provide us with sufficient evidence to determine causality. In general, the findings of the available studies do not fit with our clinical experience nor is the magnitude of the association sufficient to result in a major change in our practice. Nevertheless, the recent literature has resulted in our careful reevaluation of PPI use across both FDA indications and in general.
- ↑ Kia L, Kahrilas PJ (2016). "Therapy: Risks associated with chronic PPI use - signal or noise?". Nature Reviews. Gastroenterology & Hepatology. 13 (5): 253–4. doi:10.1038/nrgastro.2016.44. PMID 27006255. S2CID 19207074. Archived from the original on 2019-06-20. Retrieved 2018-04-20.
- ↑ Sakai, Hideki; Fujii, Takuto; Takeguchi, Noriaki (2016). "Chapter 13. Proton-Potassium (H+/K+) ATPases: Properties and Roles in Health and Diseases". In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel (eds.). The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. Vol. 16. Springer. pp. 459–483. doi:10.1007/978-4-319-21756-7_13 (inactive 2021-01-14).
{{cite book}}: CS1 maint: DOI inactive as of January 2021 (link) - ↑ Hatlebakk JG, Katz PO, Camacho-Lobato L, Castell DO (2000). "Proton pump inhibitors: better acid suppression when taken before a meal than without a meal". Aliment Pharmacol Ther. 14 (10): 1267–72. doi:10.1046/j.1365-2036.2000.00829.x. PMID 11012470. S2CID 36206292.
- ↑ AstraZeneca Pty Ltd. Nexium (Australian approved prescribing information). North Ryde: AstraZeneca; 2005.
- ↑ Wyeth Australia Pty Ltd. Zoton (Australian approved prescribing information). Baulkham Hills: Wyeth; 2004.
- ↑ Shin, Jai Moo; Munson, Keith; Vagin, Olga; Sachs, George (2008). "The gastric HK-ATPase: Structure, function, and inhibition". Pflügers Archiv: European Journal of Physiology. 457 (3): 609–22. doi:10.1007/s00424-008-0495-4. PMC 3079481. PMID 18536934.
- ↑ Carlsson, E; Lindberg, P. (2002). "Two of a kind". Chemistry in Britain. 38 (5): 42–5.
- ↑ Kim HK, Park SH, Cheung DY, Cho YS, Kim JI, Kim SS, et al. (2010). "Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects". Journal of Gastroenterology and Hepatology. 25 (10): 1618–25. doi:10.1111/j.1440-1746.2010.06408.x. PMID 20880169. S2CID 41932174.
- ↑ "Archive copy" (PDF). Archived (PDF) from the original on 2020-12-23. Retrieved 2020-12-23.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Strand, Daniel S.; Kim, Daejin; Peura, David A. (15 January 2017). "25 Years of Proton Pump Inhibitors: A Comprehensive Review". Gut and Liver. 11 (1): 27–37. doi:10.5009/gnl15502. PMC 5221858. PMID 27840364.
External links
- "Proton pump inhibitors". MedlinePlus Medical Encyclopedia. Archived from the original on 2020-01-14. Retrieved 2020-01-14.
| Classification |
|
|---|---|
| Identifiers |