Sin Nombre orthohantavirus
| Sin Nombre orthohantavirus | |
|---|---|
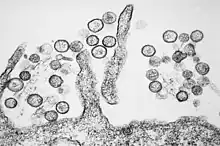 | |
| Transmission electron micrograph of Sin Nombre orthohantavirus | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Ellioviricetes |
| Order: | Bunyavirales |
| Family: | Hantaviridae |
| Genus: | Orthohantavirus |
| Species: | Sin Nombre orthohantavirus |
| Member viruses | |
| Synonyms | |
| |
| Sin Nombre virus infection | |
|---|---|
| Specialty | Virology |
Sin Nombre orthohantavirus (SNV) (from Spanish, meaning "without a name"), a member of the genus Orthohantavirus, is the prototypical etiologic agent of hantavirus cardiopulmonary syndrome (HCPS).[1]
Discovered in 1993 near the Cañon de la Muerte on the Navajo Reservation, it was originally named the Muerto Canyon hantavirus, in keeping with the convention for naming new pathogens.[2] However, the Navajo Nation objected to the name in 1994.[3] It was also near the Four Corners point in the United States, so the virologists then tried naming it the "Four Corners virus". The name was changed after local residents raised objections.[4] In frustration, the virologists changed it to Sin Nombre, meaning "without a name" in Spanish.
Virus sequencing
As with other Orthohantavirus species, SNV has a tripartite single-stranded negative-sense RNA genome. The entire genomic sequence of SNV has subsequently been determined by using RNA extracted from autopsy material as well as RNA extracted from cell culture-adapted virus. The L RNA is 6562 nucleotides (nt) in length; the M RNA is 3696 nt long; and the S RNA is 2059 to 2060 nt long. When the prototype sequence (NMH15) of SNV detected in tissues from an HPS case was compared with the sequence of the SNV isolate (NMR11; isolated in Vero E6 cells from Peromyscus maniculatus trapped in the residence of the same case), only 16 nucleotide changes were found, and none of these changes resulted in alterations in amino acid sequences of viral proteins. It had been assumed that in the process of adaptation to cell culture, selection of SNV variants which grow optimally in cell culture would occur, and selected variants would differ genetically from the parental virus. Though NMH10 and NMR11 are identical in protein sequence, nucleotide substitutions in nontranslated regions of the genome could be responsible for altered viral phenotypes, as could changes in protein glycosylation or virus membrane components.
The nested RT-PCR assay developed during the initial HCPS outbreak provided a rapid method for the genetic characterization of novel hantaviruses that did not require a virus isolate. Numerous new hantaviruses have been detected by RT-PCR in rodent tissues but have yet to be associated with human disease. These include El Moro Canyon virus associated with the western harvest mouse, Reithrodontomys megalotis, Tula virus with Microtus arvalis and M. rossiaemeridionalis, Rio Segundo virus with the Mexican harvest mouse, R. mexicanus, Isla Vista virus with the California vole, M. californicus, and Prospect Hill-like viruses in Microtus species.
Virion morphology
In contrast to members of the Orthohantavirus genus endemic outside of the Americas, whose virions are predominately round or pleomorphic, SNV virions have a greater propensity for tubular and irregular virion morphologies. This finding suggests that the genus is more diverse in terms of morphology than previously assumed, which may help explain differences in epidemiology between species. Within the Sin Nombre species, morphologic variability exists between strains, with virions of an elongated phenotype associated with higher virulence. Sin Nombre virions have an average diameter of 90 nm for round particles and 85 nm for tubular particles, with an average length of 180 nm for tubular particles, making them somewhat smaller than closely related members of the genus. [5]
History
It was first isolated in 1993 from rodents collected near the home of one of the initial patients with hantavirus pulmonary syndrome (HPS) in the Four Corners region of the western United States. Isolation was achieved through a blind passage in Peromyscus maniculatus (deer mouse) and subsequent adaptation to growth in Vero E6 cells. Additional viral strains have also been isolated from P. maniculatus associated with a fatal case in California and P. leucopus from the vicinity of probable infection of a New York case. Black Creek Canal virus was isolated from S. hispidus collected near the residence of a human case in Dade County, Florida. Another etiologic agent of HCPS, Bayou virus, was first isolated from the vicinity of Monroe, Louisiana.[6]
Epidemiology
SNV occurs wherever its reservoir rodent carrier, the deer mouse Peromyscus maniculatus,[7] is found, which includes essentially the entire populated area of North America, except for the far southeastern region from eastern Texas through Florida, Alaska, and the far northern reaches of Canada. SNV and HCPS are especially common in western states; peak incidences for HCPS have been reported in regions in which there is a lot of contact between humans and mice (New Mexico, Arizona) and in states with exceptionally large rural populations such as California. All of the western provinces of Canada have also reported cases. SNV can be contracted through the inhalation of virus-contaminated deer mouse excreta.
While transmission from the deer mouse carrier to humans is understood to occur primarily through contact with mouse urine and feces, transmission within the vector population is believed to occur through direct contact, in contrast to the understood vector transmission for other species in the Orthohantavirus genus. [8]
The case fatality ratio of SNV-induced HCPS in the USA was reported to be about 66.7% (CDC, 1993). However, since that time the case fatality ratio has steadily declined as more mild cases came to be recognized. By 2007 the CFR had declined to about 35%.
See also
References
- ↑ Ye C, Prescott J, Nofchissey R, Goade D, Hjelle B (March 2004). "Neutralizing antibodies and Sin Nombre virus RNA after recovery from hantavirus cardiopulmonary syndrome". Emerging Infect. Dis. 10 (3): 478–82. doi:10.3201/eid1003.020821 (inactive 31 December 2022). PMC 3322788. PMID 15109416.
{{cite journal}}: CS1 maint: DOI inactive as of December 2022 (link) - ↑ Van Hook, Charles J. (November 2018). "Hantavirus Pulmonary Syndrome—The 25th Anniversary of the Four Corners Outbreak". Emerging Infectious Diseases. 24 (11): 2056–2060. doi:10.3201/eid2411.180381. PMC 6199996.
- ↑ "Navajos Decry Muerto Canyon Hantavirus Site". Los Angeles Times. April 24, 1994. Archived from the original on 2019-07-03. Retrieved 3 July 2019.
- ↑ Strauss, Ellen G.; Strauss, James H. (2002). Viruses and human disease. Boston: Academic Press. p. 161. ISBN 978-0-12-673050-0.
- ↑ Parvate, Amar (2019-09-16). "Diverse Morphology and Structural Features of Old and New World Hantaviruses". Viruses. 11 (9): 862. Archived from the original on 2023-05-01. Retrieved 2023-04-02.
- ↑ "Hantaviruses, with emphasis on Four Corners Hantavirus". Bvs.insp.mx. Archived from the original on 2013-04-20. Retrieved 2016-11-17.
- ↑ Lehmer EM, Clay CA, Pearce-Duvet J, St Jeor S, Dearing MD (March 2008). "Differential regulation of pathogens: the role of habitat disturbance in predicting prevalence of Sin Nombre virus". Oecologia. 155 (3): 429–39. Bibcode:2008Oecol.155..429L. doi:10.1007/s00442-007-0922-9. PMID 18064494. S2CID 19495085.
- ↑ Warner, Bryce (2019-12-02). "Development and Characterization of a Sin Nombre Virus Transmission Model in Peromyscus maniculatus". Viruses. 11 (2): 183. Archived from the original on 2023-05-01. Retrieved 2023-04-02.
External links
| Classification |
|---|
- Virology - CDC Hantaviruses Archived 2012-02-08 at the Wayback Machine
- Clinical and Lab Recognition and other info