Torovirus
| Torovirus | |
|---|---|
.png.webp) | |
| Electron micrograph of virion and structure of equine torovirus | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Nidovirales |
| Suborder: | Tornidovirineae |
| Family: | Tobaniviridae |
| Subfamily: | Torovirinae |
| Genus: | Torovirus |
| Species | |
| |
Torovirus is a genus of enveloped, positive-strand RNA viruses in the order Nidovirales and family Tobaniviridae.[1] They primarily infect vertebrates, especially cattle, pig, and horse.[2] Diseases associated with this genus include gastroenteritis,[2] which commonly presents in mammals.[3] Torovirus is the only genus in the monotypic subfamily Torovirinae.[4] Torovirus is also a monotypic taxon, containing only one subgenus, Renitovirus.[5]
The discovery of the first torovirus can be traced back to 1970s. Equine torovirus (EToV) was accidentally found in the rectal sample from a horse who was suffering from severe diarrhea. The ‘Breda’ bovine torovirus was later found in 1979 while investigation in a dairy farm in Breda. They had several calves suffering from severe diarrhea for months. In 1984, torovirus-like particles were detected with electron microscope (EM) technique in the human patients with gastroenteritis.[6]
Virology
Structure
Toroviruses (ToV) are single-stranded RNA viruses that have a peplomer-bearing envelope that is often correlated with the enteric infections in cattle and possibly humans. These viruses appear to occur globally, occurrence of ToVs have been reported from countries in various continents like Europe, Americas, New Zealand and South Africa.[7] Torovirus particles typically possess a helical and symmetrical nucleocapsid that is coiled into a hollow cylindrical shape. The diameter is approximately 23 nm with an average length of 104 nm, where every turn cycle is at intervals of 4.5 nm.[6] ToVs are pleomorphic, with size ranging from 100 nm to 150 nm with club-like outgrowths extending from the capsid.[8][9] A nucleocapsid that is doughnut-shaped with helical symmetry is also discovered in Toroviruses.[2][10] Among various species of the Toroviruses, only equine torovirus (EToV) can be grown in the cell culture medium due to which EToVs have been most extensively studied. The information from immunofluorescence studies and morphology of the bovine Toroviruses (BToV) intestinal cells has shown similarities among the EToVs and BToVs.[11] Torovirus share some common characteristics with members of the related family Coronaviridae as they are round, pleomorphic, enveloped viruses about 120 to 140 nm in diameter.[10][12]
Genome
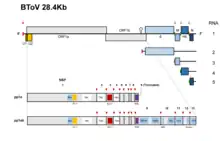
Toroviruses have a single piece of single-stranded, positive-sense RNA. The total length is approximately 25-30kb and they possess a complex replication mechanisms that includes the use of subgenomic mRNA, ribosomal frameshifting, and polymerase stuttering.[13] The genomic length of bovine torovirus is approximately 28.5 kb that is mainly comprised by one gene, replicase gene (20.2 kb). This gene contains two open reading frames, ORF1a and ORF1b that encodes proteins pp1a and pp1b.[14] The first genomic sequence of the PToV (porcine torovirus) was done in Shanghai, China in 2014. Genome length of porcine Torovirus was found to be 28301 bp and possess 79% sequence identity with the bovine Torovirus genome.[15]
Life cycle
Cattle, pig, and horse serve as the natural host of Toroviruses and infection is thought to be via the faecal-oral route.[2]
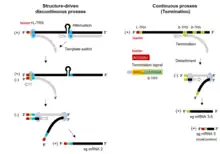
The Torovirus genome encodes for various structural proteins like spikes (S), membrane proteins (M), haemagglutinin‐esterase (HE) and nucleocapsid (N) which provides them the structural characterization necessary for infection and completing their life cycle.[16] The first three proteins coding regions are deleted in the BEV species which enables them to grow in the cell culture, while other species are unable to grow in cell culture.[17] The initial two-thirds of the torovirus genome has two open reading frames, ORF1a and ORF1b. ORFb1 encloses a domain that encodes for the enzymes such as RNA dependent RNA polymerase (RdRp) and the helicase (Hel) that are necessary for transcription and translation of the virus.[18]
Life cycle of Toroviruses involves replication within the infected host cell. These viruses predominantly bud into the lumen of the Golgi cisternae in the cytoplasm. EToV can be seen within various parts of the Golgi apparatus and in the extracellular regions as well.[19] The nucleocapsids of these viruses in the extracellular region has characteristic torus shape, whereas these nucleocapsids are rod shaped prior to budding to the host cell, which points to some morphological change happening in the maturation stage of the virus. Moreover, the nucleocapsids of the BToV and EToV are also found to accumulate in the nucleus of the infected cell.[20]
Clinical signs and diagnosis
In cattle, the disease causes diarrhoea and systemic signs such as pyrexia, lethargy and anorexia. In calves, it may cause neurological signs and lead to death in some cases. Many diagnostic techniques for torovirus infection in clinical specimens are now available such as hemagglutination (HA), enzyme-linked immunosorbent assay (ELISA), immune electron microscopy, hemagglutination-inhibition tests (HA/HI), and nucleic acid hybridization.[21] Mostly torovirus infecting humans are probably closely related to BRV or BEV and relate to any previous history of some enteric disease or infection. It is also the reason that the molecular techniques are considered more promising than the serological assays. Pigs can be infected without showing any signs and symptoms that are physically visible.
Diagnosis of the viral infection involves electron microscopy, ELISA or haemagglutination inhibition.
Treatment and control
Supportive treatment may be given to prevent dehydration and secondary infections. Control relies on good biosecurity measures including prompt isolation and disinfection of premises. As torovirus infections usually interrelates with severe diarrhea, it often led to dehydration. The most common treatment includes giving liquid therapies to the younger patients as the infection is most common in younger human population as well as in cattle. There are no as such specific preventive measures for the Torovirus infection control. Good hygiene and biosecurity practices are effective in prevention of the torovirus infection. Moreover, Antibody-containing colostrum can be given to the patients with estimated dose of 500ml/day.[22] The natural course of infection mostly researched is done in the cattle as the virus origin is related to the enteric disease in the cattle. In an experiment associated with the infection of cattle gave the results that the virus commonly transmit itself under conventional conditions. The initial symptoms in the calves showed the development of diarrhea in first 2 to 3 days of the infection. Most of the calves had the mild dehydration and a few developed a mild fever. However, none of the cases required any therapeutic intervention and were treated usually with the changes in the diet of the infected calves.[23] A research by (Vanopdenbosch et al., 1992a) published a study that torovirus respiratory infections emerges mainly in the first month of life and from 4 to 6 months of age in the autumn season peak. In all those infections, about 25% infections lead to sudden death. Besides other causes like diarrhea, pneumonia and respiratory problems, central nervous system related symptoms have also been reported in some incidental cases.[24] Studies also reveals that the young calves infected with the torovirus need to be given fluid therapies as their body cold face severe dehydration during the infection. However, the adults recover by their immune response without any external treatment if any additional infections are not present. Many researchers also suggest that the disinfection and heat sterilization could easily destroy the virus but no scientific data or reports of such results are available so far.
Human torovirus
In 1984, torovirus like particles were observed in human patients suffering from gastroenteritis or severe diarrhea.[25][26] A number of case studies then started to emerge from various parts of the world. Torovirus like particles (ToVL) was reported in scientific studies from USA, France, The Netherlands, Canada, Great Britain, India and Brazil.[27] The presence of the ToVL was reported mainly in children and adults with severe diarrhea. The term human torovirus (HuTV) is often used to describe the ToVL particles.
Because there is much similarity in HuTV and BoTV, there are certain criterion that is followed in the detecting and differentiation of both strains. Numerous studies have been conducted in the past to relate the toroviruses and its pathogenicity. Toroviruses has been found in various intestinal diseases in the children as well as the adults. A study of fecal excretion of Torovirus concluded that out of 206 examined cases, in around 72 (35%) cases the torovirus was found. As compared to the infections by rotavirus or torovirus, Toroviruses were more frequently found in the people that are more immunocompromised. The torovirus infections were characterized by reduced vomiting and increased bloody diarrhea. The immune system's antibody response mainly developed in the grown-up children who were non-immunocompromised.[28]
In addition to gastroenteritis, toroviruses has also been found in the infants with necrotizing enterocolitis.[29] However, in the same study, the severity of illness and mortality was not much affected in the patients with torovirus as compared to the patients who tested negative for torovirus.
Antigenic properties and pathogenicity
Bovine toroviruses are proposed to have mainly two different serotypes: bovine torovirus serotype 1 (BoTV-1) and bovine torovirus serotype 2 (BoTV-2).[30] Both serotypes of BoTV possess a hemagglutinin that reacts with erythrocytes from mice and rats, but not with human erythrocytes. The BoTV does not elute from rat erythrocytes after 90 min at 36 °C. Both the serotypes of the bovine Torovirus possess a hemagglutinin that reacts with red blood cells in the rodents usually.[31] No evidence so far suggests that reaction with the human erythrocytes. Although, many recent studies revealed the presence of the torovirus in humans associated with many other enteric infections, diarrhea and conditions like gastroenteritis.
The exact mechanism by which the virus induces diarrhea is currently unknown, but the studies reveal that it could be due to infection and death of cells in the small intestine and villi crypts as well as the surface crypt enterocytes in the large intestine. It is also said that the watery diarrhea could be due to lesions in the colon that lead to reduction of water absorption by the cells in the large intestine. The pathogenicity of the torovirus presence has been widely studied and explored in the bovine species, especially calves in the initial stage of life around 4 to 6 months of age.[31] Once the BoTV has been inoculated by an animal orally or nasally, it infects the epithelial cells of the villi and then extends to the components of the digestive system like the large intestine in the crypts of jejunum, ileum and colon. It ultimately results in diarrhea with in 24–72 hours of infection.[32][33] The antigens of BToV has also been reported in the dome epithelial cells and Microfold cells, present in Gut-associated lymphoid tissue of the Peyer's patch in small intestine.[34] Some researchers suggest that the BToV only infects absorptive enterocytes. However, there are researchers that also suggest that the viral replication could start in the immature epithelial cells of the crypts and can further spread to the villi.[35]
History
The discovery of the first torovirus can be traced back to 1970s. Equine torovirus (EToV) was accidentally found in the rectal sample from a horse who was suffering from severe diarrhea. The ‘Breda’ bovine torovirus was later found in 1979 while investigation in a dairy farm in Breda. They had several calves suffering from severe diarrhea for months. In 1984, torovirus-like particles were detected with electron microscope (EM) technique in the human patients with gastroenteritis.[6] In 1972, a virus was isolated from a horse in Berne, Switzerland. The virus did not react with antisera against known equine viruses and was shown to have a unique morphology and substructure.[36] In 1982 a similar, unclassified virus was isolated from calves in Breda, Iowa.[37] In 1984 particles resembling these viruses were discovered in the faeces of humans.[25] A new family of viruses—Toroviridae—was proposed at the 6th International Congress on the Taxonomy of Viruses in 1984, at Sendai, Japan.[38] However, the family is currently assigned as family Tobaniviridae with subfamily Torovirinae, order Nidovirales.[39]
Taxonomy
Until recently, the toroviruses were not assigned any family. The recent molecular analysis of the virus revealed its similarities with Arterivirus and coronaviruses, which led to the inclusion of the Torovirus along with the Arterivirus in the previously monogeneric Coronaviridae.[40] At present, toroviruses are included in the order Nidovirales sub family Torovirinae, family Tobaniviridae. Resemblance, molecular and genetic similarities, virion dimensions, behavioral ties and other characteristic similarities and differences are observed by the researchers for the taxonomic classification of the virus. In the toroviruses, the Berne virus has been extensively studied at the molecular level as compared to its other members. In 1992, the International Committee on Taxonomy of Viruses ICTV got enough data to consider torovirus in the coronavirus family due to the similarities in structure, replication behavior and the genetic sequencing.
Evolution
Toroviruses, Coronaviruses and arteriviruses are in the order Nidovirales, a group of non segmented, positive sense single stranded animal viruses.[41] Despite the differences in their genome size, varying host range and their replication strategies, the similarities in the sequence identities of the replicase proteins among different species of order Nidovirales ensures their common ancestry.[42] Sequence analysis and comparison of the polymerase and helicase domains of coronaviruses and toroviruses reveled 40-45% identical amino acids in both families, providing evidences of large evolutionary distance between the two families.[43]
References
- ↑ "ICTV Taxonomy history: Torovirus". International Committee on Taxonomy of Viruses (ICTV). ICTV. Retrieved 7 September 2020.
- 1 2 3 4 "Viral Zone". ExPASy. Retrieved 15 June 2015.
- ↑ Desk Encyclopedia of General Virology. Boston: Academic Press. 2009. p. 507. ISBN 978-0-12-375146-1.
- ↑ Virus Taxonomy: 2018 Release, International Committee on Taxonomy of Viruses, retrieved 7 December 2018
- ↑ Virus Taxonomy: 2018 Release, International Committee on Taxonomy of Viruses, retrieved 7 December 2018
- 1 2 3 Cho, K. O., & Hoet, A. E. (2014). Toroviruses (Coronaviridae). Reference Module in Biomedical Sciences, B978-0-12-801238-3.02674-X. https://doi.org/10.1016/B978-0-12-801238-3.02674-X
- ↑ A.E. Hoet, M.C. Horzinek, Torovirus, Editor(s): Brian W.J. Mahy, Marc H.V. Van Regenmortel, Encyclopedia of Virology (Third Edition), Academic Press, 2008, Pages 151-157, ISBN 9780123744104, https://doi.org/10.1016/B978-012374410-4.00516-1.
- ↑ Weiss M, Horzinek M. The proposed family Toroviridae: agents of enteric infections Arch Virol, 92 (1987), p. 1
- ↑ Koopmans M, Horzinek M. Toroviruses of animals and humans(a review) Adv Virus Res, 43 (1994), p. 233
- 1 2 ICTVdB Management (2006). 03.019.0.02. Torovirus. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA
- ↑ Hoet, A. E., & Horzinek, M. C. (2008). Torovirus. Encyclopedia of Virology, 151–157. https://doi.org/10.1016/B978-012374410-4.00516-1
- ↑ "Viral Zone". ExPASy. Retrieved 15 June 2015.
- ↑ Snijder EJ, Horzinek MC (November 1993). "Toroviruses: replication, evolution and comparison with other members of the coronavirus-like superfamily" (PDF). J. Gen. Virol. 74 (11): 2305–16. doi:10.1099/0022-1317-74-11-2305. PMID 8245847. Retrieved 11 July 2020.
- ↑ Draker R, Roper RL, Petric M, Tellier R. The complete sequence of the bovine torovirus genome. Virus Res. 2006 Jan;115(1):56-68. doi: 10.1016/j.virusres.2005.07.005. Epub 2005 Aug 30. PMID 16137782; PMCID: PMC7114287.
- ↑ Sun H, Lan D, Lu L, Chen M, Wang C, Hua X. Molecular characterization and phylogenetic analysis of the genome of porcine torovirus. Arch Virol. (2014) 159:773–8. doi: 10.1007/s00705-013-1861-x
- ↑ Snijder, E.J., and Horzinek, M.C. (1993) Toroviruses: replication, evolution and comparison with other members of the coronavirus‐like superfamily. J Gen Virol 74: 2305–2316.
- ↑ Snijder, E.J., den Boon, J.A., Horzinek, M.C., and Spaan, W.J. (1991) Comparison of the genome organization of toro‐ and coronaviruses: evidence for two nonhomologous RNA recombination events during Berne virus evolution. Virology 180: 448–452.
- ↑ Gorbalenya, A.E., Enjuanes, L., Ziebuhr, J., and Snijder, E.J. (2006) Nidovirales: evolving the largest RNA virus genome. Virus Res 117: 17–37.
- ↑ Horzinek M. C. (1999). TOROVIRUSES (CORONAVIRIDAE). Encyclopedia of Virology, 1798–1803. https://doi.org/10.1006/rwvi.1999.0285
- ↑ Eric J. Snijder and Marian C. Horzinek. 1993. Toroviruses: replication, evolution and comparison with other members of the coronavirus-like superfamily. Journal of General Virology.74, 2305-2316.
- ↑ Koopmans, M., Herrewegh, A., & Horzinek, M. C. (1991). Diagnosis of torovirus infection. Lancet, 337(8745), 859. https://doi.org/10.1016/0140-6736(91)92573-k
- ↑ Woode GN (1990). Breda virus. In: Dinter Z and Morein B, editors. Virus Infections of Ruminants. 3rd edn. Sweden: Elsevier Science, pp. 311–316
- ↑ Bosch, A., Rosa M. Pintó, and Abad, Xavier. June, 2013.Survival and Transport of Enteric Viruses in Environment. http://www.ub.edu/virusenterics/wp-content/uploads/2013/06/GOY6.pdf
- ↑ Vanopdenbosch, E., Wellemans, G., Oudewater, J., nd Petroff, k. (1992a). Vlaams Di-ergennesk. Tijdschr. 61, 1-7.
- 1 2 Beards GM, Hall C, Green J, Flewett TH, Lamouliatte F, Du Pasquier P (May 1984). "An enveloped virus in stools of children and adults with gastroenteritis that resembles the Breda virus of calves". Lancet. 1 (8385): 1050–2. doi:10.1016/S0140-6736(84)91454-5. PMC 7173266. PMID 6143978.
- ↑ Beards GM, Brown DW, Green J, Flewett TH (September 1986). "Preliminary characterisation of torovirus-like particles of humans: comparison with Berne virus of horses and Breda virus of calves". Journal of Medical Virology. 20 (1): 67–78. doi:10.1002/jmv.1890200109. PMC 7166937. PMID 3093635.
- ↑ Hoet, A., & Saif, L. (2004). Bovine torovirus (Breda virus) revisited. Animal Health Research Reviews, 5(2), 157-171. doi:10.1079/AHR200498
- ↑ Frances B. Jamieson, Elaine E. L. Wang, Cindy Bain, Jennifer Good, Lynn Duckmanton, Martin Petric, Human Torovirus: A New Nosocomial Gastrointestinal Pathogen, The Journal of Infectious Diseases, Volume 178, Issue 5, November 1998, Pages 1263–1269, https://doi.org/10.1086/314434
- ↑ Lodha, A., de Silva, N., Petric, M. and Moore, A.M. (2005), Human torovirus: A new virus associated with neonatal necrotizing enterocolitis. Acta Pædiatrica, 94: 1085-1088. doi:10.1111/j.1651-2227.2005.tb02049.x
- ↑ Woode GN, Mohammed KA, Saif LJ, Winand NJ, Quesada M, Kelso NE and Pohlenz JF (1983). Diagnostic methods for the newly discovered ‘Breda’ group of calf enteritis inducing viruses. In: Proceedings of the Third International Symposium of Veterinary Laboratory Diagnosticians, Volume 2, pp. 533–538. Ames, USA.
- 1 2 Woode GN, Reed DE, Runnels PL, Herrig MA and Hill HT (1982). Studies with an unclassified virus isolated from diarrheic calves. Veterinary Microbiology 7: 221–240.
- ↑ Pohlenz JFL, Cheville NF, Woode GN and Mokresh AH (1984). Cellular lesions in intestinal mucosa of gnotobiotic calves experimentally infected with a new unclassified bovine virus (Breda virus). Veterinary Pathology 21: 407–417
- ↑ Hall GA (1987). Comparative pathology of infection by novel diarrhoea viruses. In: Brock G and Whelan J, editors. Novel Diarrhoea Viruses. Ciba Foundation Symposium, No. 128. Chichester: John Wiley & Sons, pp. 192–217
- ↑ Pohlenz JFL, Cheville NF, Woode GN and Mokresh AH (1984). Cellular lesions in intestinal mucosa of gnotobiotic calves experimentally infected with a new unclassified bovine virus (Breda virus). Veterinary Pathology 21: 407–417.
- ↑ Koopmans M and Horzinek MC (1995). The pathogenesis of torovirus infections in animals and humans. In: Siddell SG, editor. The Coronaviridae. New York: Plenum Press, pp. 403–413
- ↑ Weiss M, Steck F, Horzinek MC (September 1983). "Purification and partial characterization of a new enveloped RNA virus (Berne virus)" (PDF). J. Gen. Virol. 64 (9): 1849–58. doi:10.1099/0022-1317-64-9-1849. PMID 6886677. Retrieved 11 July 2020.
- ↑ Woode GN, Reed DE, Runnels PL, Herrig MA, Hill HT (July 1982). "Studies with an unclassified virus isolated from diarrheic calves". Vet. Microbiol. 7 (3): 221–40. doi:10.1016/0378-1135(82)90036-0. PMC 7117454. PMID 7051518.
- ↑ Horzinek MC, Weiss M (October 1984). "Toroviridae: a taxonomic proposal". Zentralblatt für Veterinärmedizin. Reihe B. 31 (9): 649–59. doi:10.1111/j.1439-0450.1984.tb01348.x. hdl:1874/3490. PMID 6393658
- ↑ "ICTV Taxonomy history: Torovirus". International Committee on Taxonomy of Viruses (ICTV). ICTV. Retrieved 7 September 2020.
- ↑ Cavanagh, D., Brien, D. A., Brinton, M., Enjuanes, L., Holmes, K. V., Horzinek, M. C., Lai, M., Laude, H., Plagemann, P., Siddell, S., Spaan, W., Taguchi, F., & Talbot, P. J. (1994). Revision of the taxonomy of the Coronavirus, Torovirus and Arterivirus genera. Archives of virology, 135(1-2), 227–237. https://doi.org/10.1007/BF01309782
- ↑ Ivanov K.A., Hertzig T., Rozanov M., Bayer S., Thiel V., Gorbalenya A.E., Ziebuhr J. Major genetic marker of nidoviruses encodes a replicative endoribonuclease. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12694–12699
- ↑ Phylogenetic and Evolutionary Relationships among Torovirus Field Variants: Evidence for Multiple Intertypic Recombination Events, S. L. Smits, A. Lavazza, K. Matiz, M. C. Horzinek, M. P. Koopmans, R. J. de Groot Journal of Virology Aug 2003, 77 (17) 9567-9577; DOI: 10.1128/JVI.77.17.9567-9577.2003
- ↑ Snijder, E. J., and M. C. Horzinek. 1993. Toroviruses: replication, evolution and comparison with other members of the coronavirus-like superfamily. J. Gen. Virol.74:2305-2316
External links
- Toroviruses reviewed and published by Wikivet, accessed 08/10/2011
- Viralzone: Torovirus
- Virus Pathogen Database and Analysis Resource (ViPR): Coronaviridae
- ICTV